With increasing life expectancy for HIV-infected persons, the ravages of both AIDS-defining and non-AIDS cancers become increasingly prominent. In this issue of Blood, Stebbing et al present work that will aid not only in the diagnosis of MCD, but could lead to preventative therapy.1
Multicenteric Castleman Disease (MCD) is a rare lymphoproliferative disorder caused by the human herpes virus-8 (HHV-8)2 leading to expansion of the plasmablasts in the mantle zone of the lymph node.3 The disease is highly associated with HIV infection.4 Patients present with an acute clinical syndrome, including adenopathy, hepatosplenomegaly, fevers, sweats, peripheral neuropathy, and diminished performance status.5 It is characterized by a remitting and relapsing course. Rituximab has activity for unclear reasons,5,6 although research in optimal therapies is limited. The morbidity of the disease is significant and MCD can be fatal.
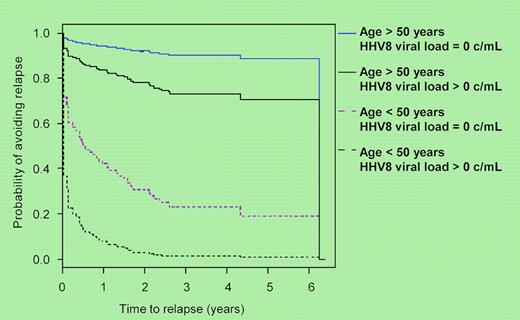
Previously, Powles et al demonstrated that more than 80% of patients with HIV-MCD had detectable plasma HHV-8 viremia compared with 36% of HIV seropositive patients with Kaposi sarcoma, 3% with lymphoma, and none of 53 HIV-positive control patients.7 Building on this, Stebbing et al studied 52 patients with MCD by obtaining plasma samples at least every 3 months measuring HHV-8 DNA levels by PCR C-reactive protein.1 Admittedly, treatment was not entirely uniform, although 42 patients received rituximab-based therapy, with (n = 14) or without (n = 28) etoposide. They did well, with 45 achieving remission and 31 remaining disease free. The median follow-up of 49 months strengthens the study, as does the number of samples, 190 during active disease and 420 in remission. In patients with detectable HHV-8 viral loads (> 50 copies/mL) during remission, the risk of relapse was significantly higher, hazard ratio 2.9 (95% confidence interval: 1.3-6.7) than those with undetectable HHV-8. Moreover, patients younger than 50 years with detectable HHV8 viral loads had a 80% to 90% relapse rate in 1 to 2 years, respectively (see figure). Those with undetectable viral loads had a 60% relapse at 1 year. For patients older than 50 years, corresponding relapse rates were 10% to 20%. The reason for the age discrepancy is entirely unclear.
The authors suggest that HHV-8 levels be used in both the diagnostic criteria for MCD and be considered as a tool to predict relapse. The latter will be important if a preventive treatment can be identified. Candidates include rituximab and anti-IL6,8 as the latter is highly associated with MCD. Notably, 53% of the patients in this study had undetectable HIV viral loads. The CD4 counts were non–AIDS-defining in half of the patients. This is consistent with an overall good prognosis from the HIV perspective and warrants an aggressive approach to MCD that can lead to long-term survival, much in the way we approach HIV/AIDS-related lymphoma.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal