Platelet secretion of dense granule contents is important for human hemostasis, as is evident from the mucocutaneous bleeding associated with disorders of dense granule biogenesis and secretion.1 Dense granule secretion is also important for growth and stability of platelet thrombi in experimental models of thrombosis.2 Although patients with primary defects in platelet secretion make up a large proportion of those with hereditary platelet function disorders, the underlying molecular cause is rarely identified.3 Thus, understanding molecular mechanisms regulating platelet secretory function continues to be an important area of research.
It has been clear for years that the protein kinase C (PKC) family is important for platelet secretory function. PKCs are a family of serine-threonine kinases, at least 4 to 5 of which are expressed in human platelets (α, β, δ, θ and possibly ν).4 Of these, PKCα was recently shown to be particularly important for dense granule secretion5 ; however, the substrates of PKCα and their specific roles in granule secretion have remained murky. In this issue of Blood, Konopatskaya et al identify protein kinase D2 (PKD2) as a substrate of PKCα in platelets and demonstrate the importance of this serine-threonine kinase for thrombus formation on collagen in a flow system.6
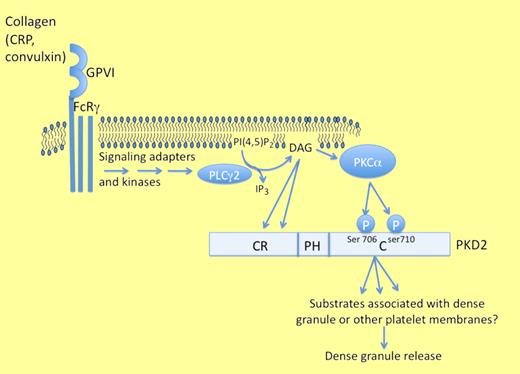
The human protein kinase Ds (PKD1, PKD2, and PKD3) share a common structure composed of a cysteine-rich domain containing 2 diacylglycerol (DAG) binding sites at the N-terminus, a central pleckstrin homology (PH) domain, and a C-terminal catalytic domain. In lymphocytes, activation of PKD is achieved by a combination of DAG binding to the cysteine-rich domain and phosphorylation of 2 serine residues in the catalytic domain, commonly termed 744 and 748 based on the sequence of PKD1.7 Thus, in PKD-expressing cells, liberation of DAG from the plasma membrane by phospholipase C may serve to colocalize PKDs at lipid membrane sites together with active PKCs, allowing activation by PKC-dependent phosphorylation of residues 744 and 748. Because phosphorylation of residues 744 and 748 (or the homologous residues 706/710 in PKD2 and 731/735 in PKD3) controls the kinase activity of the kinase,8 mutagenesis of these 2 sites renders the kinase inactive. By knocking out specific PKD isoforms in mice or knocking in the kinase-dead mutants of these isoforms, it has been established that PKD1 is important for embryogenesis and pathologic cardiac remodeling to stress stimuli,9 while PKD2 is required for optimal peripheral T lymphocyte responses.10 The results of the PKD3 knockout have not yet been published. By comparing platelets from mice expressing kinase-dead mutant isoforms of PKDs 1 or 2, Konopatskaya et al establish that PKD2 is the important PKD isoform for regulating platelet dense granule secretion and thrombus formation ex vivo.6 A graphic summary of the proposed role of PKD2 in platelet signaling and granule secretion is shown in the figure.
Konopatskaya et al demonstrate that protein kinase C–dependent activation of protein kinase D2 (PKD2) is required for optimal platelet dense granule secretion and aggregation and regulates thrombus formation on collagen surfaces.6
Konopatskaya et al demonstrate that protein kinase C–dependent activation of protein kinase D2 (PKD2) is required for optimal platelet dense granule secretion and aggregation and regulates thrombus formation on collagen surfaces.6
Stafford et al have previously shown that PKD becomes phosphorylated and activated after stimulation of human platelets with thrombin or convulxin, but the functional significance of its activation was not known.11 Here, Konopatskaya et al show that platelets from mice expressing the kinase-dead mutant form of PKD2 have suboptimal aggregation responses to thrombin and collagen-related peptide.6 Strikingly, ATP release after stimulation by either agonist is reduced by nearly half in the knockin platelets and thrombus growth on collagen-coated surfaces is also dramatically reduced. The defect in aggregation in the kinase-dead PKD2 knockin platelets was corrected by addition of exogenous ADP, suggesting that a deficiency in ADP secretion is in fact responsible for the aggregation defect. However, the loss of PKD2 activity only modestly affects aggregation, while the effect on thrombus formation under flow conditions is more robust; this may be because of the different contribution of secretion to these two models of platelet-platelet contact, but it remains formally possible that PKD plays an additional secretion-independent role in thrombus formation under flow.
This paper adds an important link in the chain of events leading from PKC activation in platelets to dense granule secretion. Many questions remain: Is some fraction of the many patients with unknown causes of mucocutaneous bleeding attributable to PKD signaling defects in platelets? Given that tail bleeding times were normal in the PKD2 kinase-dead mice, but thrombus growth was impaired, is PKD2 an attractive target for development of antithrombotic therapeutics? Experiments using PKCα−/− platelets clearly demonstrate that PKCα regulates phosphorylation of PKD after CRP stimulation, but there was little or no defect in PKD phosphorylation induced by thrombin in the PKCα−/− platelets: is another PKC family member compensating under these conditions? More importantly, what are the substrates of PKD and how do they regulate the mechanics of dense granule trafficking and release? Answering these questions may give us new insights into the roles of PKD in the secretory processes of platelets and perhaps other cells.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal