Abstract
Symptomatic burden in myeloproliferative neoplasms is present in most patients and compromises quality of life. We sought to validate a broadly applicable 18-item instrument (Myeloproliferative Neoplasm Symptom Assessment Form [MPN-SAF], coadministered with the Brief Fatigue Inventory) to assess symptoms of myelofibrosis, essential thrombocythemia, and polycythemia vera among prospective cohorts in the United States, Sweden, and Italy. A total of 402 MPN-SAF surveys were administered (English [25%], Italian [46%], and Swedish [28%]) in 161 patients with essential thrombocythemia, 145 patients with polycythemia vera, and 96 patients with myelofibrosis. Responses among the 3 administered languages showed great consistency after controlling for MPN subtype. Strong correlations existed between individual items and key symptomatic elements represented on both the MPN-SAF and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30. Enrolling physicians' blinded opinion of patient symptoms (6 symptoms assessed) were highly correlated with corresponding patients' responses. Serial administration of the English MPN-SAF among 53 patients showed that most MPN-SAF items are well correlated (r > 0.5, P < .001) and highly reproducible (intraclass correlation coefficient > 0.7). The MPN-SAF is a comprehensive and reliable instrument that is available in multiple languages to evaluate symptoms associated with all types of MPNs in clinical trials globally.
Introduction
Myeloproliferative neoplasms (MPNs), that is, essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF), are a group of MPNs that can lead to significant rates of morbidity and mortality among afflicted patients. Specifically, patients in the early stages of these illnesses (ET, PV, and early MF) can be predisposed to thrombohemorrhagic events and vascular complications. Long-term complications of MPNs (either primary MF or MF arising from an antecedent ET or PV) can include progressive cytopenias, constitutional symptoms, cachexia and weight loss, moderate to massive splenomegaly, and risk of blastic transformation.
Symptomatic burden in MPN is severe and present in most persons with disease. We previously conducted an online survey of 1179 patients with MPN to quantify the symptomatic burden of illness, with results indicating that constitutional symptoms and symptoms associated with splenomegaly were prominent (present in 70% of patients) and compromised quality of life.1 Additional complaints included fatigue (81%), pruritus (52%), night sweats (49%), bone pain (44%), fever (14%), and weight loss (13%). Most often patients experienced profound fatigue in excess of age-matched controls. Many patients required assistance for activities of daily living or disability (34.5%) and reported MPN-associated disability (11.2%).1 The presence of similar constitutional symptoms have been recently included as a negative predictor of survival in the prognostic scoring system established by the International Working Group for Myelofibrosis Research and Treatment.2 Also of recent clinical significance, trials of newly developed JAK2 inhibitors have achieved significant reductions in MF-associated symptoms,3 with promising data seen in PV and ET as well.4 Given the potential of JAK2 inhibitors to palliate MPN symptom burden and with 10 JAK2 inhibitors currently undergoing clinical trials, an accurate and reliable measure of MPN symptoms profile will be an essential component for our understanding of these compound's clinical benefits.
We previously validated the Myelofibrosis Symptom Assessment Form (MF-SAF)5 ; however, utility was limited to patients with MF and did not adequately capture the spectrum of microvascular symptoms common in patients with ET and PV. In addition, we recognized that patients with ET or PV can acquire MF-associated symptoms to a variable degree over the course of their illness, even before they have met the formal criteria for MF after ET/PV.6 Therefore, we subsequently created the Myeloproliferative Symptom Assessment Form (MPN-SAF) to function as a single instrument of patient-reported symptoms for the entire spectrum of patients with MPN. In addition, because the goal of the creation and validation of the MPN-SAF is to have a tool useful for measurement of MPN symptomatic response in international trials, we sought to validate this expanded instrument with the use of both concurrent measures of disease and internal reliability in the United States, Italy, and Sweden.
Methods
Initial survey administration: MPN-SAF
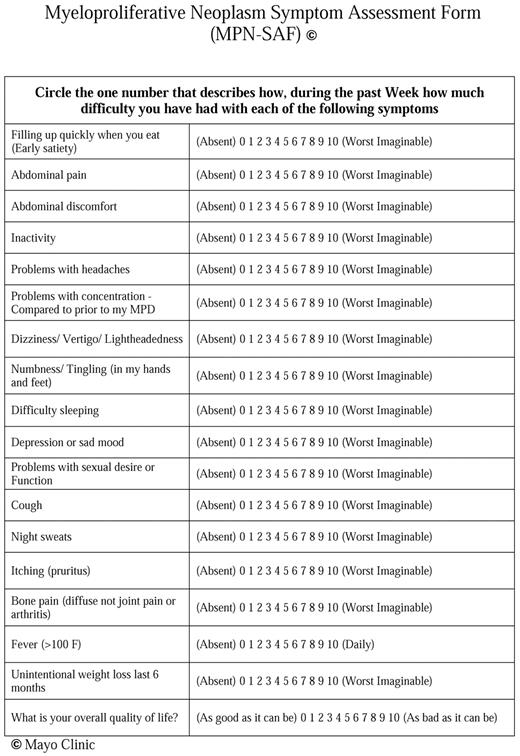
On the basis of data from patients with ET and PV 1 we modified the previously validated MF-SAF5 to include 7 additional questions (Figure 1). Symptoms chosen to be included in the survey were based on an Internet survey of patients with MPN.1 Fatigue was independently measured by coadministration of the previously published Brief Fatigue Inventory (BFI; copyright M. D. Anderson Cancer Center).7 In addition to assessing fatigue, early satiety, abdominal pain, abdominal discomfort, inactivity, cough, night sweats, pruritus, bone pain, fever, weight loss, and quality of life, the expanded survey asked patients to rate “Problems with headaches,” “Problems with concentration—compared with before my MPD,” “Dizziness/Vertigo/Lightheadedness,” “Numbness/Tingling (in my hands and feet),” “Difficulty sleeping,” “Depression or sad mood,” and “Problems with sexual desire or function” on a scale from 0 (absent/as good as it can be) to 10 (worst-imaginable/as bad as it can be).
The Myelofibrosis Symptom Assessment Form (MPN-SAF) Validation Survey.
Translations
The survey was developed in English and translated into alternative languages by an established Patient Reported Outcome (PRO) translation method.8 This translation technique uses 3 independent survey translations created with the use of translators fluent in both the original and translating languages. Survey translations are then compared by a fourth translator who works with other translators to develop a consensus translation. Translated languages included Swedish and Italian.
MPN-SAF validation
Once survey development was complete, patients were accrued prospectively from a variety of practice settings, including private practice, academic, and government-funded medical centers that included the Mayo Clinic in Scottsdale, Arizona; Ospedali Riuniti di Bergamo in Bergamo, Italy, University of Pavia in Pavia, Italy, University of Florence in Florence, Italy, NU Hospital in Uddevalla, Sweden, Stockholm South Hospital in Stockholm, Sweden, and Uppsala University Hospital in Uppsala, Sweden, between November 2009 and August 2010. This protocol was approved in all participating institution's institutional review boards before implementation, and informed consent was obtained from participants before study involvement.
Patients self-completed the MPN-SAF and the accompanying European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (EORTC QLQ-C30,9 a widely used PRO instrument for patients with cancer) during a physician office visit for MPN disease in the language respective to their region of office visit. After survey completion, patients were asked to assess whether the survey was easy to understand and addressed most of their symptoms. Patients were also asked to include any additional symptoms not included in the survey in an open-ended question. Physicians blinded to the patient's responses were asked to rank (0-10 scale) patient's symptoms of night sweats, fevers, bone pain, pruritus, weight loss, fatigue, and quality of life. In addition, physicians were asked to indicate the patient's current and prior MPN therapies, date of MPN diagnosis, spleen size, and available laboratory values, including hemoglobin level, platelet count, white blood cell count, absolute neutrophil count, and percentage of blasts if present.
Serial survey administration
Patients who participated in the initial MPN-SAF survey in English were sent a repeat survey and modified letter of consent by US mail. Participants were asked to complete the survey and return it in a self-addressed, stamped envelope. Persons completing the serial survey were also asked to assess demographic variables, including ethnicity, education, and marital status.
Data analysis
Categorical variables were compared between groups with the use of χ2 tests. Continuous variables were compared between groups with the use of ANOVA F tests or 2-sample t tests. Pearson correlations were computed with P values to assess relationships between pairs of variables with absolute Pearson correlation values > .5 being considered as a moderate level of correlation. General linear models were used to compare patient scores between language groups while adjusting for disease type. For serially administered data, Pearson correlations were again computed to assess relationships between time points. In addition, the intraclass correlation coefficient (ICC) was computed on the basis of a 2-way ANOVA model. Statistical software used included SAS software Version 9 (SAS Institute). P values < .05 were considered statistically significant throughout except where noted.
Results
Patients
Four hundred two MPN-SAF surveys were compiled (including surveys in English [n = 102; 25%], Italian [n = 186; 46%], and Swedish [n = 114; 28%]) and analyzed for characteristics of disease. Compiled data included 161 patients with ET (40%), 145 patients with PV (36%), and 96 patients with MF (24%) with an average of 7.8 years (range, 0-43 years) from the diagnosis of their MPN (Table 1). Participants were of typical age (64.9 years; range, 26-91 years) and sex (53% female) characteristic of disease. Prior hemorrhage (10%) and thrombosis (25%) were frequent. Average spleen size was 2 cm below the costal margin, with 1.5% of patients having undergone a prior splenectomy. The majority of patients currently received cytoreductive therapy (68%) or received cytoreductive therapy in the past (84%), with hydroxyurea being most common.
Demographic characteristics of participants by language
| . | Italian (n = 186) . | English (n = 102) . | Swedish (n = 114) . | Total (N = 402) . | P . |
|---|---|---|---|---|---|
| MPN type, n (%) | < .001* | ||||
| ET | 88 (47.3) | 20 (19.6) | 53 (46.5) | 161 (40) | |
| PV | 69 (37.1) | 23 (22.5) | 53 (46.5) | 145 (36.1) | |
| MF | 29 (15.6) | 59 (57.8) | 8 (7.0) | 96 (23.9) | |
| MPN duration, y | NS | ||||
| Mean (SD) | 7.6 (6.8) | 8.2 (9.1) | 7.7 (7.1) | 7.8 (7.5) | |
| Range | 0.0-29.0 | 0.0-43.0 | 0.0-42.0 | 0.0-43.0 | |
| Age, y | .004† | ||||
| Mean (SD) | 62.9 (12.2) | 65.2 (12.5) | 67.8 (11.7) | 64.9 (12.3) | |
| Range | 29.0-91.0 | 26.0-89.0 | 27.0-88.0 | 26.0-91.0 | |
| Sex | NS | ||||
| F, n (%) | 103 (55.4) | 50 (49) | 60 (52.6) | 213 (53.0) |
| . | Italian (n = 186) . | English (n = 102) . | Swedish (n = 114) . | Total (N = 402) . | P . |
|---|---|---|---|---|---|
| MPN type, n (%) | < .001* | ||||
| ET | 88 (47.3) | 20 (19.6) | 53 (46.5) | 161 (40) | |
| PV | 69 (37.1) | 23 (22.5) | 53 (46.5) | 145 (36.1) | |
| MF | 29 (15.6) | 59 (57.8) | 8 (7.0) | 96 (23.9) | |
| MPN duration, y | NS | ||||
| Mean (SD) | 7.6 (6.8) | 8.2 (9.1) | 7.7 (7.1) | 7.8 (7.5) | |
| Range | 0.0-29.0 | 0.0-43.0 | 0.0-42.0 | 0.0-43.0 | |
| Age, y | .004† | ||||
| Mean (SD) | 62.9 (12.2) | 65.2 (12.5) | 67.8 (11.7) | 64.9 (12.3) | |
| Range | 29.0-91.0 | 26.0-89.0 | 27.0-88.0 | 26.0-91.0 | |
| Sex | NS | ||||
| F, n (%) | 103 (55.4) | 50 (49) | 60 (52.6) | 213 (53.0) |
Significant (P < .05), based on a χ2 test across all groups.
Significant (P < .05), based on an ANOVA F test.
On average, patients took ∼ 7-13 minutes to complete the survey packet, of which the MPN-SAF took ∼ 5 minutes. No patients refused to participate in the survey after giving consent; however, 3% (12 of 402) of patients did not complete ≥ 1 page of the survey. Uncompleted pages were most often in the middle of the packet, suggesting that they were missed by participants.
Symptomatic burden
Patients reported that symptoms associated with MPN disease were severe and frequent among all 3 MPNs. Consistent with previous findings, disease burden was greatest in patients with MF, followed by patients with PV, then patients with ET (Table 2). Fatigue (as measure by the BFI) was the most common symptom (93% prevalence), with highest average severity of all reported symptoms (mean score of 3.2). Other common findings included decreased quality of life (84%), insomnia (65%), sad mood (65%), and sexuality problems (58%). The least common symptoms (< 50% prevalence) were fevers (20%), weight loss (35%), abdominal pain (46%), cough (46%), headache (48%), and bone pain (49%). Although symptoms are present in all 3 MPN subgroups, itching is notably more burdensome in patients with PV (65%; median score of 2.8 of 10). In addition, abdominal pain, abdominal discomfort, early satiety, and inactivity all are most prevalent and severe in MF. Interestingly, night sweats (present in 56%) overall had similar prevalence and severity across all 3 MPNs.
Patient's assessment of symptom severity and percent of patients that had symptoms by disease type
| . | ET (n = 161) . | PV (n = 145) . | MF (n = 96) . | Total (N = 402) . | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) . | Prevalence, % . | Mean (95% CI) . | Prevalence, % . | Mean (95% CI) . | Prevalence, % . | Mean (95% CI) . | Prevalence . | |
| Fatigue (BFI score) | 2.9 (2.5-3.3) | 90.3 | 3.0 (2.6-3.4) | 91.7 | 3.7 (3.3-4.2) | 98.9 | 3.2 (2.9-3.4) | 92.7 |
| Early satiety | 2.0 (1.6-2.4) | 53.2* | 2.3 (1.9-2.8) | 62.1 | 2.7 (2.2-3.2) | 75.5* | 2.3 (2.0-2.5) | 61.9 |
| Abdominal pain | 1.1 (0.8-1.4)* | 38.2* | 1.2 (0.8-1.5)† | 43.0 | 2.4 (1.8-3.0)*† | 63.0* | 1.4 (1.2-1.7) | 45.9 |
| Abdominal discomfort | 1.6 (1.2-2.0)* | 45.3* | 1.6 (1.2-2.0)† | 49.3† | 3 (2.4-3.6)*† | 71.7*† | 2.0 (1.7-2.2) | 53.2 |
| Inactivity | 1.7 (1.3-2.1)* | 53.7* | 1.9 (1.5-2.4) | 57.9 | 3.0 (2.4-3.5)† | 76.5* | 2.1 (1.8-2.4) | 60.5 |
| Headache | 1.3 (1.0-1.7) | 47.1 | 1.4 (1.1-1.8) | 52.2 | 1.5 (1.0-2.0) | 44.6 | 1.4 (1.2-1.6) | 48.3 |
| Concentration problem | 2.1 (1.6-2.5) | 55.8 | 2.3 (1.8-2.7) | 61.2 | 3.0 (2.4-3.6) | 72.5 | 2.4 (2.1-2.6) | 61.7 |
| Dizziness | 2.0 (1.6-2.4) | 56.1 | 1.8 (1.4-2.2) | 52.1 | 2.0 (1.4-2.5) | 58.1 | 1.9 (1.7-2.2) | 55.2 |
| Numbness | 2.3 (1.8-2.7) | 58.8 | 2.6 (2.1-3.0) | 66.2 | 2.4 (1.8-3.0) | 58.1 | 2.4 (2.1-2.7) | 61.3 |
| Insomnia | 2.6 (2.1-3.0) | 58.0 | 3.0 (2.5-3.5) | 68.1 | 3.1 (2.5-3.7) | 73.9 | 2.8 (2.5-3.1) | 65.4 |
| Sad mood | 2.2 (1.8-2.7) | 57.3 | 2.2 (1.7-2.6) | 65.0 | 2.3 (1.8-2.8) | 68.5 | 2.2 (2.0-2.5) | 62.7 |
| Sexuality problems | 2.5 (2.0-3.0) | 51.0 | 2.8 (2.2-3.4) | 56.8 | 4.0 (3.2-4.7) | 71.0 | 3.0 (2.6-3.3) | 57.9 |
| Cough | 1.6 (1.2-2.0) | 41.4 | 1.3 (1.0-1.6) | 45.7 | 1.7 (1.2-2.2) | 55.4 | 1.5 (1.3-1.7) | 46.4 |
| Night sweats | 2.2 (1.8-2.7) | 51.3 | 2.3 (1.8-2.7) | 57.4 | 2.4 (1.8-3.0) | 63.4 | 2.3 (2.0-2.6) | 56.4 |
| Itching | 1.7 (1.3-2.2) | 40.6‡ | 2.8 (2.3-3.3) | 65.0‡ | 1.9 (1.4-2.5) | 53.8 | 2.2 (1.9-2.5) | 52.6 |
| Bone pain | 1.9 (1.5-2.4) | 45.2 | 2.1 (1.6-2.6) | 47.5 | 2.2 (1.6-2.8) | 55.3 | 2.0 (1.7-2.3) | 48.5 |
| Fever | 0.4 (0.2-0.6) | 17.0 | 0.3 (0.1-0.4) | 17.9 | 0.6 (0.3-0.9) | 29.0 | 0.4 (0.3-0.5) | 20.2 |
| Weight loss | 0.8 (0.5-1.1)* | 23.4* | 1.1 (0.7-1.5) | 36.2 | 1.9 (1.3-2.5)* | 48.9* | 1.2 (0.9-1.4) | 34.2 |
| Quality of life | 2.9 (2.5-3.4) | 76.8* | 3.1 (2.7-3.4) | 85.5 | 3.6 (3.2-4.1) | 94.7* | 3.1 (2.9-3.4) | 84.2 |
| . | ET (n = 161) . | PV (n = 145) . | MF (n = 96) . | Total (N = 402) . | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) . | Prevalence, % . | Mean (95% CI) . | Prevalence, % . | Mean (95% CI) . | Prevalence, % . | Mean (95% CI) . | Prevalence . | |
| Fatigue (BFI score) | 2.9 (2.5-3.3) | 90.3 | 3.0 (2.6-3.4) | 91.7 | 3.7 (3.3-4.2) | 98.9 | 3.2 (2.9-3.4) | 92.7 |
| Early satiety | 2.0 (1.6-2.4) | 53.2* | 2.3 (1.9-2.8) | 62.1 | 2.7 (2.2-3.2) | 75.5* | 2.3 (2.0-2.5) | 61.9 |
| Abdominal pain | 1.1 (0.8-1.4)* | 38.2* | 1.2 (0.8-1.5)† | 43.0 | 2.4 (1.8-3.0)*† | 63.0* | 1.4 (1.2-1.7) | 45.9 |
| Abdominal discomfort | 1.6 (1.2-2.0)* | 45.3* | 1.6 (1.2-2.0)† | 49.3† | 3 (2.4-3.6)*† | 71.7*† | 2.0 (1.7-2.2) | 53.2 |
| Inactivity | 1.7 (1.3-2.1)* | 53.7* | 1.9 (1.5-2.4) | 57.9 | 3.0 (2.4-3.5)† | 76.5* | 2.1 (1.8-2.4) | 60.5 |
| Headache | 1.3 (1.0-1.7) | 47.1 | 1.4 (1.1-1.8) | 52.2 | 1.5 (1.0-2.0) | 44.6 | 1.4 (1.2-1.6) | 48.3 |
| Concentration problem | 2.1 (1.6-2.5) | 55.8 | 2.3 (1.8-2.7) | 61.2 | 3.0 (2.4-3.6) | 72.5 | 2.4 (2.1-2.6) | 61.7 |
| Dizziness | 2.0 (1.6-2.4) | 56.1 | 1.8 (1.4-2.2) | 52.1 | 2.0 (1.4-2.5) | 58.1 | 1.9 (1.7-2.2) | 55.2 |
| Numbness | 2.3 (1.8-2.7) | 58.8 | 2.6 (2.1-3.0) | 66.2 | 2.4 (1.8-3.0) | 58.1 | 2.4 (2.1-2.7) | 61.3 |
| Insomnia | 2.6 (2.1-3.0) | 58.0 | 3.0 (2.5-3.5) | 68.1 | 3.1 (2.5-3.7) | 73.9 | 2.8 (2.5-3.1) | 65.4 |
| Sad mood | 2.2 (1.8-2.7) | 57.3 | 2.2 (1.7-2.6) | 65.0 | 2.3 (1.8-2.8) | 68.5 | 2.2 (2.0-2.5) | 62.7 |
| Sexuality problems | 2.5 (2.0-3.0) | 51.0 | 2.8 (2.2-3.4) | 56.8 | 4.0 (3.2-4.7) | 71.0 | 3.0 (2.6-3.3) | 57.9 |
| Cough | 1.6 (1.2-2.0) | 41.4 | 1.3 (1.0-1.6) | 45.7 | 1.7 (1.2-2.2) | 55.4 | 1.5 (1.3-1.7) | 46.4 |
| Night sweats | 2.2 (1.8-2.7) | 51.3 | 2.3 (1.8-2.7) | 57.4 | 2.4 (1.8-3.0) | 63.4 | 2.3 (2.0-2.6) | 56.4 |
| Itching | 1.7 (1.3-2.2) | 40.6‡ | 2.8 (2.3-3.3) | 65.0‡ | 1.9 (1.4-2.5) | 53.8 | 2.2 (1.9-2.5) | 52.6 |
| Bone pain | 1.9 (1.5-2.4) | 45.2 | 2.1 (1.6-2.6) | 47.5 | 2.2 (1.6-2.8) | 55.3 | 2.0 (1.7-2.3) | 48.5 |
| Fever | 0.4 (0.2-0.6) | 17.0 | 0.3 (0.1-0.4) | 17.9 | 0.6 (0.3-0.9) | 29.0 | 0.4 (0.3-0.5) | 20.2 |
| Weight loss | 0.8 (0.5-1.1)* | 23.4* | 1.1 (0.7-1.5) | 36.2 | 1.9 (1.3-2.5)* | 48.9* | 1.2 (0.9-1.4) | 34.2 |
| Quality of life | 2.9 (2.5-3.4) | 76.8* | 3.1 (2.7-3.4) | 85.5 | 3.6 (3.2-4.1) | 94.7* | 3.1 (2.9-3.4) | 84.2 |
Comparisons of prevalence were evaluated with χ2 tests. Comparisons of severity were evaluated with 2-sample t tests.
Significant difference between ET and MF (P < .001).
Significant difference between PV and MF (P < .001).
Significant difference between ET and PV (P < .001).
Comparison across populations
Administration of the surveys among an international sample showed great consistency between both sampled languages and countries. Data initially indicated that English survey respondents (recruited in the United States) reported greater symptom severity for most items. However, after controlling for MPN subtype, of which English surveys had the highest proportion of MF (Table 1), symptom data between the 3 languages were similar for all items except overall quality of life (P = .004), worst fatigue (P = .02), pruritus (P = .004), and bone pain (P = .01). Itching, bone pain, and decreased quality of life were rated as most severe in Italian patients (2.7, 2.5, and 3.5, respectively, with greater scores indicating higher severity). However, worst fatigue had the highest prevalence in English respondents (39.2% rated fatigue as 7-10 of 10 compared with 18.5% for Italian and 29.8% for Swedish respondents, significant even after controlling for disease type).
Comparison to EORTC QLQ-C30
Strong correlations existed between individual items represented on both the MPN-SAF and the EORTC QLQ-C30, including pain, fatigue (BFI), appetite, and insomnia (all P < .001; Table 3). In addition, key symptomatic elements were highly correlated with the EORTC QLQ-C30 functional subscales. Comparison of average EORTC scores indicated a high correlation between the current study's data and results of previously published trials (Table 4). EORTC scores for MF closely matched previous published data for recurrent/metastatic cancer and acute myelocytic leukemia (AML), whereas scores for PV and ET more closely corresponded with age-matched controls, consistent with previous observations.10
Correlations between relevant scales of the EORTC-QLQ C30 and MPN SAF (N = 402)
| MPN-SAF item . | EORTC QLQ-C30 subscale . | Correlation . | EORTC QLQ-C30 symptom scale . | Correlation . |
|---|---|---|---|---|
| Overall QOL | Physical function Role function Global health status/QOL | 0.51 0.55 0.52 | Fatigue | 0.57 |
| BFI (mean) | Physical function Role function Emotional function Cognitive function Social Function Global health status/QOL | 0.66 0.61 0.51 0.50 0.54 0.61 | Fatigue Pain Dyspnea | 0.75 0.56 0.51 |
| BFI worst fatigue | Physical function Role function Global health status/QOL | 0.56 0.50 0.52 | — | |
| Concentration | Role function Cognitive function | 0.52 0.65 | Fatigue | 0.59 |
| Inactivity | Physical function | 0.51 | Fatigue | 0.56 |
| Sad mood | Emotional function | 0.72 | Fatigue | 0.53 |
| Abdominal discomfort | Role function | 0.55 | — | |
| Abdominal pain | — | Pain | 0.55 | |
| Bone pain | — | Pain | 0.57 | |
| Insomnia | — | Insomnia | 0.77 | |
| Early satiety | — | Appetite loss | 0.52 |
| MPN-SAF item . | EORTC QLQ-C30 subscale . | Correlation . | EORTC QLQ-C30 symptom scale . | Correlation . |
|---|---|---|---|---|
| Overall QOL | Physical function Role function Global health status/QOL | 0.51 0.55 0.52 | Fatigue | 0.57 |
| BFI (mean) | Physical function Role function Emotional function Cognitive function Social Function Global health status/QOL | 0.66 0.61 0.51 0.50 0.54 0.61 | Fatigue Pain Dyspnea | 0.75 0.56 0.51 |
| BFI worst fatigue | Physical function Role function Global health status/QOL | 0.56 0.50 0.52 | — | |
| Concentration | Role function Cognitive function | 0.52 0.65 | Fatigue | 0.59 |
| Inactivity | Physical function | 0.51 | Fatigue | 0.56 |
| Sad mood | Emotional function | 0.72 | Fatigue | 0.53 |
| Abdominal discomfort | Role function | 0.55 | — | |
| Abdominal pain | — | Pain | 0.55 | |
| Bone pain | — | Pain | 0.57 | |
| Insomnia | — | Insomnia | 0.77 | |
| Early satiety | — | Appetite loss | 0.52 |
Absolute Pearson correlations of > .50 were chosen as a cutoff to represent a moderate level of correlation. For all items, P < .001.
QOL indicates quality of life.
Comparison of EORTC subscales compared to established norms
| . | EORTC QLQ-C30 Scores (mean ± SD) . | ||||||
|---|---|---|---|---|---|---|---|
| Current data . | Samuelsson 200610 . | EORTC 200811 . | |||||
| ET (n = 161) . | PV (n = 145) . | MF (n = 96) . | PV and ET (N = 37-38) . | General population (N = 7802) . | Recurrent/metastatic cancer (N = 4812) . | AML (N = 155) . | |
| EORTC subscales | |||||||
| Physical functioning | 85.1 ± 16.9 | 83.3 ± 17.7 | 74.9 ± 20.7 | 90.6 ± 11.5 | 89.8 ± 16.2 | 75.8 ± 23.1 | N/A |
| Role functioning | 85.0 ± 23.0 | 85.2 ± 22.7 | 68.8 ± 28.9 | 81.5 ± 24.5 | 84.7 ± 25.4 | 60.7 ± 35.1 | N/A |
| Emotional functioning | 77.7 ± 22.9 | 78.2 ± 20.8 | 76.5 ± 20.5 | 82.4 ± 17.1 | 76.3 ± 22.8 | 68.7 ± 24.8 | 82.2 ± 18.9 |
| Cognitive functioning | 83.4 ± 19.4 | 83.0 ± 18.8 | 77.0 ± 20.4 | 86.7 ± 17.4 | 86.1 ± 20.0 | 80.5 ± 23.2 | 86.1 ± 18.5 |
| Social functioning | 88.4 ± 19.7 | 88.3 ± 20.1 | 74.9 ± 24.1 | 89.6 ± 18.6 | 87.5 ± 22.9 | 70.5 ± 30.7 | 66.1 ± 31.0 |
| Global health status/QOL | 71.1 ± 24.9 | 65.7 ± 24.8 | 59.9 ± 24.6 | 72.1 ± 23.4 | 71.2 ± 22.4 | 56.3 ± 25.6 | N/A |
| EORTC symptom scales | |||||||
| Fatigue | 26.9 ± 25.5 | 29.3 ± 21.9 | 41.0 ± 25.1 | 23.1 ± 24.1 | 24.1 ± 24.0 | 41.8 ± 29.4 | 36.2 ± 22.7 |
| Nausea/vomiting | 4.0 ± 9.7 | 3.3 ± 8.2 | 6.3 ± 11.4 | 2.6 ± 6.2 | 3.7 ± 11.7 | 13.1 ± 22.5 | 9.0 ± 18.3 |
| Pain | 14.3 ± 23.1 | 14.6 ± 20.4 | 22.6 ± 27.8 | 15.4 ± 25.2 | 20.9 ± 27.6 | 33.7 ± 32.4 | 13.7 ± 20.4 |
| Dyspnea | 18.5 ± 25.8 | 19.6 ± 24.2 | 29.8 ± 29.0 | 10.6 ± 15.7 | 11.8 ± 22.8 | 23.4 ± 30.1 | 11.3 ± 17.1 |
| Insomnia | 25.0 ± 27.4 | 26.6 ± 28.0 | 33.7 ± 30.6 | 19.3 ± 27.5 | 21.8 ± 29.7 | 33.6 ± 33.4 | 20.4 ± 26.1 |
| Appetite loss | 5.1 ± 14.7 | 10.3 ± 21.7 | 15.1 ± 23.1 | 2.6 ± 9.1 | 6.7 ± 18.3 | 28.2 ± 34.9 | 18.0 ± 30.5 |
| Constipation | 19.0 ± 29.0 | 13.4 ± 24.5 | 16.8 ± 26.1 | 4.4 ± 13.8 | 6.7 ± 18.4 | 23.2 ± 32.3 | 7.9 ± 19.1 |
| Diarrhea | 9.0 ± 18.3 | 6.3 ± 16.3 | 21.1 ± 27.1 | 4.4 ± 11.4 | 7.0 ± 18.0 | 10.7 ± 22.4 | 12.6 ± 25.1 |
| Financial difficulties | 8.0 ± 19.3 | 6.4 ± 15.9 | 17.5 ± 28.7 | 7.2 ± 17.8 | 9.5 ± 23.3 | 16.2 ± 27.7 | 18.7 ± 28.8 |
| . | EORTC QLQ-C30 Scores (mean ± SD) . | ||||||
|---|---|---|---|---|---|---|---|
| Current data . | Samuelsson 200610 . | EORTC 200811 . | |||||
| ET (n = 161) . | PV (n = 145) . | MF (n = 96) . | PV and ET (N = 37-38) . | General population (N = 7802) . | Recurrent/metastatic cancer (N = 4812) . | AML (N = 155) . | |
| EORTC subscales | |||||||
| Physical functioning | 85.1 ± 16.9 | 83.3 ± 17.7 | 74.9 ± 20.7 | 90.6 ± 11.5 | 89.8 ± 16.2 | 75.8 ± 23.1 | N/A |
| Role functioning | 85.0 ± 23.0 | 85.2 ± 22.7 | 68.8 ± 28.9 | 81.5 ± 24.5 | 84.7 ± 25.4 | 60.7 ± 35.1 | N/A |
| Emotional functioning | 77.7 ± 22.9 | 78.2 ± 20.8 | 76.5 ± 20.5 | 82.4 ± 17.1 | 76.3 ± 22.8 | 68.7 ± 24.8 | 82.2 ± 18.9 |
| Cognitive functioning | 83.4 ± 19.4 | 83.0 ± 18.8 | 77.0 ± 20.4 | 86.7 ± 17.4 | 86.1 ± 20.0 | 80.5 ± 23.2 | 86.1 ± 18.5 |
| Social functioning | 88.4 ± 19.7 | 88.3 ± 20.1 | 74.9 ± 24.1 | 89.6 ± 18.6 | 87.5 ± 22.9 | 70.5 ± 30.7 | 66.1 ± 31.0 |
| Global health status/QOL | 71.1 ± 24.9 | 65.7 ± 24.8 | 59.9 ± 24.6 | 72.1 ± 23.4 | 71.2 ± 22.4 | 56.3 ± 25.6 | N/A |
| EORTC symptom scales | |||||||
| Fatigue | 26.9 ± 25.5 | 29.3 ± 21.9 | 41.0 ± 25.1 | 23.1 ± 24.1 | 24.1 ± 24.0 | 41.8 ± 29.4 | 36.2 ± 22.7 |
| Nausea/vomiting | 4.0 ± 9.7 | 3.3 ± 8.2 | 6.3 ± 11.4 | 2.6 ± 6.2 | 3.7 ± 11.7 | 13.1 ± 22.5 | 9.0 ± 18.3 |
| Pain | 14.3 ± 23.1 | 14.6 ± 20.4 | 22.6 ± 27.8 | 15.4 ± 25.2 | 20.9 ± 27.6 | 33.7 ± 32.4 | 13.7 ± 20.4 |
| Dyspnea | 18.5 ± 25.8 | 19.6 ± 24.2 | 29.8 ± 29.0 | 10.6 ± 15.7 | 11.8 ± 22.8 | 23.4 ± 30.1 | 11.3 ± 17.1 |
| Insomnia | 25.0 ± 27.4 | 26.6 ± 28.0 | 33.7 ± 30.6 | 19.3 ± 27.5 | 21.8 ± 29.7 | 33.6 ± 33.4 | 20.4 ± 26.1 |
| Appetite loss | 5.1 ± 14.7 | 10.3 ± 21.7 | 15.1 ± 23.1 | 2.6 ± 9.1 | 6.7 ± 18.3 | 28.2 ± 34.9 | 18.0 ± 30.5 |
| Constipation | 19.0 ± 29.0 | 13.4 ± 24.5 | 16.8 ± 26.1 | 4.4 ± 13.8 | 6.7 ± 18.4 | 23.2 ± 32.3 | 7.9 ± 19.1 |
| Diarrhea | 9.0 ± 18.3 | 6.3 ± 16.3 | 21.1 ± 27.1 | 4.4 ± 11.4 | 7.0 ± 18.0 | 10.7 ± 22.4 | 12.6 ± 25.1 |
| Financial difficulties | 8.0 ± 19.3 | 6.4 ± 15.9 | 17.5 ± 28.7 | 7.2 ± 17.8 | 9.5 ± 23.3 | 16.2 ± 27.7 | 18.7 ± 28.8 |
N/A indicates not available; and QOL, quality of life.
Comparison to physician perceptions
Enrolling physicians who were blinded to their respective patient's reported MPN-SAF scores assessed 6 symptoms of disease (including night sweats, fevers, fatigue [BFI], weight loss, bone pain, and pruritus). Comparison of the results indicated excellent correlation with corresponding patients' responses except bone pain (all P < .001; Table 5).
Correlation of patient scores to corresponding physician perceptions (N = 402)
| MPN-SAF item . | MD perceptions . |
|---|---|
| Fatigue (BFI score)* | 0.50 |
| Night sweats | 0.51 |
| Fever | 0.55 |
| Weight loss | 0.54 |
| Itching | 0.62 |
| Bone pain | 0.48 |
| MPN-SAF item . | MD perceptions . |
|---|---|
| Fatigue (BFI score)* | 0.50 |
| Night sweats | 0.51 |
| Fever | 0.55 |
| Weight loss | 0.54 |
| Itching | 0.62 |
| Bone pain | 0.48 |
Absolute Pearson correlations of > .50 was chosen as a cutoff to represent a moderate level of correlation. For all items, P < .001.
Patient-reported BFI score correlated to physician's assessment of fatigue.
Serial MPN-SAF results
Of patients previously administered the MPN-SAF in English, 53 patients (ET [17.6%], PV [25.5%], and MF [56.9%]) responded to a repeat MPN-SAF survey sent by US mail. Approximately 52% of participants invited to participate in the repeated survey responded, with a mean time between surveys of 190 ± 63 days (range, 43-257 days). Patients who responded to serial data were of similar demographic distribution as the larger single administration (54% female with an average age of 66.5 ± 11.7 years and mean MPN duration of 8.3 ± 9.8 years).
Pearson correlations indicate that most MPN-SAF items are well correlated (r > 0.5, P < .001) on repeat survey administration, including mean BFI, early satiety, abdominal pain, abdominal discomfort, inactivity, headache, concentration, dizziness, numbness, insomnia, sad mood, sexuality, night sweats, pruritus, and bone pain (Table 6). Items characteristic of advanced disease, including weight loss, fever, and cough, displayed lower Pearson correlations (r = 0.46, −0.08, and 0.38, respectively). ICCs for test-retest reliability indicated that common features of disease, including mean BFI, inactivity, insomnia, and night sweats, were highly reproducible on serial survey administration (ICC > 0.7).
Correlations between serial survey administrations (N = 53)
| MPN-SAF Item . | Correlation coefficient . | P . | ICC (95% CI) . |
|---|---|---|---|
| Fatigue (BFI score) | 0.74 | < .001 | 0.735 (0.573-0.842) |
| Early satiety | 0.55 | < .001 | 0.546 (0.313-0.717) |
| Abdominal pain | 0.54 | < .001 | 0.532 (0.293-0.709) |
| Abdominal discomfort | 0.54 | < .001 | 0.533 (0.295-0.709) |
| Inactivity | 0.72 | < .001 | 0.708 (0.503-0.826) |
| Headache | 0.62 | < .001 | 0.570 (0.347-0.733) |
| Concentration problem | 0.70 | < .001 | 0.691 (0.508-0.815) |
| Dizziness | 0.57 | < .001 | 0.545 (0.315-0.716) |
| Numbness | 0.69 | < .001 | 0.686 (0.502-0.810) |
| Insomnia | 0.72 | < .001 | 0.720 (0.550-0.832) |
| Sad mood | 0.57 | < .001 | 0.570 (0.346-0.734) |
| Sexuality problems | 0.68 | < .001 | 0.648 (0.449-0.786) |
| Cough | 0.38 | .01 | 0.348 (0.079-0.572) |
| Night sweats | 0.73 | < .001 | 0.724 (0.556-0.835) |
| Itching | 0.69 | < .001 | 0.686 (0.500-0.811) |
| Bone pain | 0.59 | < .001 | 0.593 (0.373-0.750) |
| Fever | 0.08 | .60 | −0.068 (−0.350 to 0.211) |
| Weight loss | 0.46 | .001 | 0.409 (0.144-0.619) |
| Quality of life | 0.59 | < .001 | 0.594 (0.376-0.750) |
| MPN-SAF Item . | Correlation coefficient . | P . | ICC (95% CI) . |
|---|---|---|---|
| Fatigue (BFI score) | 0.74 | < .001 | 0.735 (0.573-0.842) |
| Early satiety | 0.55 | < .001 | 0.546 (0.313-0.717) |
| Abdominal pain | 0.54 | < .001 | 0.532 (0.293-0.709) |
| Abdominal discomfort | 0.54 | < .001 | 0.533 (0.295-0.709) |
| Inactivity | 0.72 | < .001 | 0.708 (0.503-0.826) |
| Headache | 0.62 | < .001 | 0.570 (0.347-0.733) |
| Concentration problem | 0.70 | < .001 | 0.691 (0.508-0.815) |
| Dizziness | 0.57 | < .001 | 0.545 (0.315-0.716) |
| Numbness | 0.69 | < .001 | 0.686 (0.502-0.810) |
| Insomnia | 0.72 | < .001 | 0.720 (0.550-0.832) |
| Sad mood | 0.57 | < .001 | 0.570 (0.346-0.734) |
| Sexuality problems | 0.68 | < .001 | 0.648 (0.449-0.786) |
| Cough | 0.38 | .01 | 0.348 (0.079-0.572) |
| Night sweats | 0.73 | < .001 | 0.724 (0.556-0.835) |
| Itching | 0.69 | < .001 | 0.686 (0.500-0.811) |
| Bone pain | 0.59 | < .001 | 0.593 (0.373-0.750) |
| Fever | 0.08 | .60 | −0.068 (−0.350 to 0.211) |
| Weight loss | 0.46 | .001 | 0.409 (0.144-0.619) |
| Quality of life | 0.59 | < .001 | 0.594 (0.376-0.750) |
For ICC, values > 0.7 were considered a moderate level.
Patient's perceptions
The survey was well received by respondents and had few missed symptoms according to patient report. Patients indicated that the MPN-SAF was easy to understand (98%) and addressed most of their MPN symptoms (96%). On open response, no additional symptoms not addressed in the survey were reported more than twice. Most reported symptoms were only mentioned once and were secondary to disease processes such as splenomegaly or thrombosis.
Discussion
The MPN-SAF is a comprehensive and reliable 27-item instrument that concisely assesses the prevalence and severity of symptoms of MF, PV, and ET, with many symptoms not captured in previous instruments. Our current trial is the largest report of MPN symptoms among a prospectively accrued population to date and shows that the MPN-SAF is a valid and reliable instrument that has excellent correlations with physician's perceptions of disease and alternative measures of cancer symptoms. Measurement of fatigue by the BFI complemented the assessment of MPN-specific symptoms through the use of the MPN-SAF. The MPN-SAF displayed strong association with EORTC QLQ-C309 subscales, including physical, role, cognitive, social, emotional, and quality-of-life functioning, and with symptom scales, including specific symptoms common to malignancy. Results from serial survey administration indicate that the survey captures both the primary disease state as well as continued disease presence, even in the midst of standard therapy treatment. The survey is well suited for use in international populations, with current translations into English-, Italian-, and Swedish-speaking populations and efforts under way to develop alternative MPN-SAF translations.
In addition to being well validated, the MPN-SAF is a tailored measurement tool with its evaluation based on measures using PRO and comprehensive assessment of MPN symptom burden. PRO measurements represent a subjective assessment of self-reported symptoms and treatment effects that have been found to be useful in guiding key clinical decisions,12 particularly when objective evaluation of physical manifestations are difficult.13 Other PRO instruments developed for use in patients with solid tumors, including the EORTC QLQ-C309 and the M. D. Anderson Symptom Inventory,14 do not measure the breadth of symptoms11,15 specific to MPN disease, such as abdominal pain, bone pain, headache, pruritus, weight loss, fever, and cough, that the MPN-SAF is able to assess. Thus, previous large MPN clinical trials have not used these traditional cancer instruments because of their lack of ability to comprehensively assess MPN disease state. Our modifications to the original MF-SAF,5 namely inclusion of items assessing headaches, concentration, dizziness, numbness/tingling, insomnia, depression, and sexual dysfunction, showed that the MPN-SAF caught a large portion of all associated MPN symptoms, with all new items except for headaches present in most patients with MF, PV, or ET and with no additional symptoms identified by open-answer response.
The MPN-SAF is also a novel measurement tool to gauge MPN severity and debilitation. Previous researchers have stated that there is a paucity of data about health-related quality of life among hematologic malignancies compared with solid tumors.11,16,17 This is unfortunate because quality of life plays a critical role in MPN disease burden,1 and consideration of health-related quality of life may positively affect clinical outcomes.11,18 In addition, symptom burden has been shown to be particularly troublesome for patients with MPN, with a greatest severity in MF.1 To our knowledge, this work represents the first published comparison of EORTC QLQ-C30 data for MF compared with other related conditions (Table 4). Similar to what could be anticipated from keen clinical insight, this brief glimpse of MF indicates that the disease bears striking similarity to recurrent or metastatic cancer, most notably for EORTC scales of physical functioning, quality of life, fatigue, and financial difficulties. Closely related, AML carried a less severe symptom profile, which may be accounted for by the inclusion of de novo AML, which is less aggressive than AML secondary to prior malignancy or therapy.19 Data for ET and PV were consistent with a much less severe symptom profile, more closely resembling that of a generally sampled population. However, it may be that the EORTC did not capture the full spectrum of problems prevalent in PV and ET.
With the emergence of the modern era of JAK2 inhibitor therapy,20 notably with the ongoing clinical trials of JAK2 inhibitors INCB018424,3 TG101348,21 SB1518,22 and CYT387,23 which have been effective in reducing night sweats, itching, abdominal pain/discomfort, and bone and muscle pain, as well as splenomegaly and anemia (CYT387 only), it is essential to have a comprehensive instrument to monitor disease-associated symptoms. Current efforts are under way to translate the MPN-SAF into French, German, Spanish, Mandarin, and Cantonese with the aim of constructing a useful and broadly available tool for MPN symptom assessment. Further studies will also evaluate the timing of the MPN-SAF in regard to serial reporting in a variety of practice settings and efficacy to detect clinically significant changes in symptoms during therapeutic trials. Thus, with the continued validation of this survey into alternative languages and treatment settings, we recommend the MPN-SAF as a uniform symptom assessment tool for patients with MPN on clinical trials globally.
Presented at the 52nd annual meeting of the American Society of Hematology, Orlando, FL, December 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Charles Cleeland, MD, and M. D. Anderson Cancer Center for the use of the Brief Fatigue Inventory along with this project. There were no additional contributors in the writing, editing, or researching of this manuscript.
Authorship
Contribution: R.A.M. coordinated the research and oversaw all aspects of the project; R.S. was responsible for survey coordination, data entry, and manuscript preparation; and A.C.D. was the project biostatistician. All other coauthors assisted with survey administration and development. All authors contributed to the authorship of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruben A. Mesa, Division of Hematology and Oncology, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ; e-mail: mesa.ruben@mayo.edu.