In this issue of Blood, Singh and colleagues identify HMG-CoA reductase-dependent farnesylation of Rac-1 as critical for 15(S)-HETE-induced angiogenesis.1 These findings establish a novel link between eicosanoid and cholesterol metabolism with important biologic and therapeutic implications for angiogenesis.
Angiogenesis plays an important role in embryonic development as well as in numerous pathologic states including malignancy, atherosclerosis, and ocular diseases.2 The discovery that angiogenesis is a key contributor to multiple disease states provided the essential goad for vigorous efforts to identify key molecular pathways mediating this process. Such efforts have not only revealed many biologic insights but have also led to the development of novel therapies directed at major pro-angiogenic stimuli such as vascular endothelial growth factor (VEGF). However, initial enthusiasm has been somewhat muted by the recognition that drugs targeting VEGF are of limited efficacy, underscoring the fact that angiogenesis is mediated by myriad of subcellular systems and that a complex interplay exists between various angiogenic factors. Although research conducted over the past several years has identified numerous anti-angiogenic molecules, much of the intracellular signaling pathways still remain to be elucidated. A better understanding of pro- and anti-angiogenic molecules and the pathways involved represent novel therapeutic strategies in the treatment of neoplasia and other vascular disorders.
Arachidonic acid (AA) is an essential fatty acid required by a majority of mammals. AA is an important component of membrane phospholipids and is released in response to agonists such as hormones and cytokines.3 A growing body of evidence suggests that eicosanoids, the metabolites of AA produced by the catalytic activities of cyclooxygenase (COX), lipoxygenase (LOX), or cytochrome P450 monooxygenase (CYP) pathways, influence angiogenesis and stimulate the proliferative capacity of various cells, highlighting their potential significance in cancer biology, atherosclerosis, and other pathologic states.4,5 The abundance of evidence identifying the various functions of AA and the eicosanoids has largely been possible because of pharmacologic inhibition studies correlating overexpression with pathologic consequences. Although inhibitors of the AA cascade are obvious attractive therapeutic options, much of the precise molecular mechanisms involved in AA-induced angiogenesis remain to be elucidated.
In the present study, Singh and colleagues establish a novel link between eicosanoids and a key enzyme in cholesterol metabolism, 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoAR).1 The importance of HMG-CoAR in clinical medicine is well established. Indeed, inhibition of this enzyme by a class of drugs called statins significantly reduces cardiovascular morbidity and mortality and remains one of the most celebrated pharmacotherapies ever developed.6 Research in the past few years has also demonstrated that HMG-CoAR inhibitors exert anti-angiogenic effects via inhibition of RhoA-dependent pathways.7
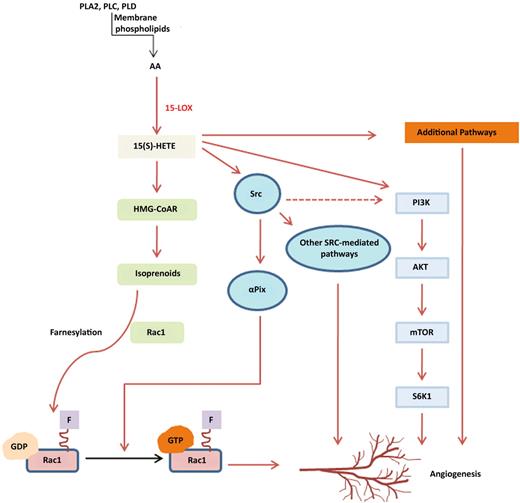
In their quest to understand how eicosanoids affect angiogenesis, Singh and colleagues have previously identified numerous pathways via which 15(S)-hydroxyeicosatetraenoic acid (HETE), a major product of AA metabolism via the 15-LOX, induces angiogenesis, including Src,8 a non-receptor tyrosine kinase, and Rac 1, a Rho GTPase,9 both of which are important regulators of endothelial migration and tube formation (see figure). Other 15(S)-HETE–mediated angiogenesis pathways so far identified involve phosphoinositide 3-kinase (PI3-K; see figure). The authors now address the signaling pathways that sustain the 15(S)-HETE–mediated activation of Rac 1. Their findings reveal a dual mechanism—one involving Src-mediated αPix activation and the second involving induction of HMG-CoAR. The latter was required for Rac-1 farnesylation and localization to the plasma membrane. Using simvastatin, an HMG-CoAR inhibitor, and mevalonate, a product of HMG-CoAR enzyme, the authors confirm the importance of HMG-CoAR as a downstream target of 15(S)-HETE. In vivo confirmation was achieved through elegant studies revealing defective angiogenesis in mice deficient in 15-HETE production (12/15-LOX null strain). Finally, the authors confirmed the anti-angiogenic effect of HMG-CoAR inhibition by statins and provided vital information regarding the mechanistic basis for this effect.
This schema illustrates the various pathways involved in 15(S)-HETE–mediated angiogenesis. Definitions of most abbreviations are in the commentary text. PLA2 indicates Phospholipase A2; PLC, Phospholipase C; PLD, Phospholipase D; mTOR, mammalian target of rapamycin; SGK1, SG Kinase-1.
This schema illustrates the various pathways involved in 15(S)-HETE–mediated angiogenesis. Definitions of most abbreviations are in the commentary text. PLA2 indicates Phospholipase A2; PLC, Phospholipase C; PLD, Phospholipase D; mTOR, mammalian target of rapamycin; SGK1, SG Kinase-1.
The limitations of the study by Singh et al should direct future research. The dose of statin used for the in vivo studies (5 mg/kg/d) is significantly higher than that used in human subjects (maximum 80 mg/d). Further, it has also been shown that low doses of statin may augment angiogenesis while higher doses inhibit this process as shown in the present study. Whether eicosanoids are involved in the effects of low-dose statins is an important area for investigation. In addition, while the authors observe that exogenous addition of mevalonate alone was insufficient to cause substantial farnesylation, it completely rescued Rac1 activation and restored blood flow in the presence of simvastatin. In addition, we note that while Rac1-specific GTP/GDP exchange factors are dependent on HMG-CoAR activity, inhibition of 15(S)-HETE only partially blocked Rac1 GTP, although farnesylation was completed suppressed. While interpretation of the discrepancy between the in vitro and in vivo results is complicated, it may suggest the involvement of other signaling pathways. Finally, how does 15-HETE induce HMG-CoAR and could this regulation, through effects on protein prenylation or cholesterol synthesis (and consequently steroidogenesis and bile salt production) underlie the broad biologic effects of eicosanoids?
To conclude, this study by Singh et al reinforces the role of eicosanoids in angiogenesis and is the first to implicate the cholesterol biosynthetic pathway as central to their effects. The findings have significant clinical implications; especially since HMG-CoAR inhibitors are already widely used in cardiovascular disorders and have a minimal side-effect profile. It is tempting to consider that the beneficial effects of statins could possibly now be extended to other medical fields such as cancer and ocular vascular disorders. Indeed, clinical studies are under way testing the effect of statins in some oncologic settings.10 The results of Singh et al's study also raise the possibility that pharmacologic inhibition of eicosanoid production could be exploited for therapeutic gain in the treatment of cancer and cardiovascular disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal