Abstract
To study factors associated with anemia and its effect on survival in HIV-infected persons treated with modern combined antiretroviral therapy (cART), we characterized the prevalence of anemia in the Veterans Aging Cohort Study (VACS) and used a candidate gene approach to identify proinflammatory gene single nucleotide polymorphisms (SNPs) associated with anemia in HIV disease. The study comprised 1597 HIV+ and 865 HIV− VACS subjects with DNA, blood, and annotated clinical data available for analysis. Anemia was defined according to World Health Organization criteria (hemoglobin < 13 g/dL and < 12 g/dL in men and women, respectively). The prevalence of anemia in HIV+ and HIV− subjects was 23.1% and 12.9%, respectively. Independent of HIV status, anemia was present in 23.4% and 8% in blacks and whites, respectively. Analysis of our candidate genes revealed that the leptin −2548 G/A SNP was associated with anemia in HIV+, but not HIV−, patients, with the AA and AG genotypes significantly predicting anemia (P < .003 and P < .039, respectively, logistic regression). This association was replicated in an independent cohort of HIV+ women. Our study provides novel insight into the association between genetic variability in the leptin gene and anemia in HIV+ individuals.
Introduction
Anemia is a common hematologic morbidity of HIV infection, and predicts for decreased survival, disease progression, and decreased quality of life.1 Anemia prevalence in persons with AIDS has been reported to range anywhere from 1% to 95%,2 invariably reflecting heterogeneity in HIV cohorts studied and the definition used to classify anemia. The etiology of anemia in HIV is multifactorial, including opportunistic infections, nutritional deficiency, malignancy, and medications.3,4 Evidence also suggests that HIV affects the differentiation of HSCs, perhaps by primary infection of myeloid progenitors, or secondary to chronic inflammation related to HIV and its secondary comorbidities.5 In addition, as the population of those infected with the HIV virus continues to age, individuals are also at risk for developing the anemia of aging, which comprises classic anemia of inflammation (AI), anemia because of nutritional deficiencies, and unexplained anemia in one-third each of anemic individuals 65 years of age or older.6 Although HIV infection is felt to promote a chronic inflammatory state, its impact on the pathogenesis of anemia subtypes in HIV disease remains to be determined.
Several studies suggest that levels of inflammatory cytokines and acute-phase proteins increase with age.7,8 However, it is controversial as to what extent this reflects primary dysregulation of the immune response or comorbid disease. A growing body of evidence has advanced our understanding of the important role of adipokines in modulating the body's energy balance as well as signaling pathways associated with propagation of a chronic inflammatory state. Since the discovery of leptin and related adipokines, it has become recognized that adipocytes are an important source of metabolic regulatory hormones with far-reaching consequences.9 Leptin functions in a feedback loop to regulate body weight through pathways that regulate hypothalamic responses to energy balance.10 Leptin is thought to have evolved to protect against starvation, and mediates multiple neuroendocrine responses to low energy balance, including suppression of fertility and a variety of metabolic changes.9 Leptin insensitivity appears to increase with age,11 and may mediate the metabolic aspects of frailty associated with aging.12 A functional polymorphism of the leptin gene at −254813 has been linked to obesity,14,15 as well as to cardiovascular16 and oncologic outcomes.17 Leptin may also impact erythropoiesis, having been shown to stimulate hematopoietic progenitor cell proliferation,18 modulate erythropoietin (EPO) sensitivity in patients with end-stage renal disease,19,20 and up-regulate the expression of hepcidin.21
Adipokines are also felt to play an important role in HIV-related disease. Leptin and adiponectin have both been implicated in combined antiretroviral therapy (cART)–related lipodystrophy and metabolic syndrome.22,23 Leptin deficiency is associated with lipoatrophy, while low levels of adiponectin are associated with central fat accumulation and insulin resistance.22 In addition to affecting metabolic pathways that lead to changes in fat distribution, leptin, and adiponectin also influence immune pathways that may both modulate HIV disease and influence BM responsiveness. Leptin induces TNFα and IL-6, and also increases neutrophil and T-cell function. Adiponectin has counterregulatory properties to these effects, inhibiting phagocytosis and adhesion of macrophages, and decreasing TNFα and IL-6 levels.23
In the present study, we examined the association of functional single nucleotide polymorphisms (SNPs) in proinflammatory genes with known effects on erythropoiesis and anemia in distinct cohorts of individuals with HIV disease. Our examination of a subgroup of the Veterans Aging Cohort Study (VACS), a longitudinal study of demographically matched veterans with and without HIV disease, revealed that the leptin −2548 G/A SNP is independently associated with anemia in HIV+, but not HIV−, patients. This association was replicated in the Swiss HIV Cohort Study. Our study provides novel insight into the association between genetic variability in the leptin gene and anemia in independent cohorts of HIV+ individuals.
Methods
Study populations
The Veterans Aging Cohort Study (VACS) is the largest HIV cohort in North America.24 It includes a nested, consented 8 site sample (VACS 8) of 3660 HIV+ veterans demographically matched to 3652 HIV− veterans (HIV−) with longitudinal in-depth data spanning nearly 10 years. The database includes full electronic medical record data and annual self-completed surveys on all subjects. The VACS also contains a large tissue repository, including DNA, plasma, and serum samples. More than 75% of VACS subjects are black or Latino and the median age is ∼ 50 years. Because the study is within the VA system, ∼ 90% of the patients are male.
The Swiss HIV Cohort Study (http://www.shcs.ch) is a prospective cohort study with continuing enrollment of HIV-infected individuals aged 16 years or older and has been approved by ethical committees of all participating institutions with written informed consent obtained from all participating individuals.
Tissue samples
Banked DNA and blood specimens from consenting subjects in VACS 8 were collected at 5 participating VACS sites under the auspice of an approved institutional review board (IRB) protocol.24 All blood tubes were processed on receipt, assigned specific DNA Bank or VACS Blood Bank numbers, and stored in −80°C freezers at the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) Core Laboratory (Boston, MA).
Definition of anemia and anemia of inflammation
Anemia was classified according to World Health Organization (WHO) criteria using hemoglobin of < 13 g/dL and 12 g/dL in men and women, respectively. Anemia of inflammation (AI) was defined as an elevated ferritin (> 170) in the presence of a low serum iron count (< 60 μg/dL) without evidence of iron deficiency.
Laboratory variables
Ferritin and transferrin were measured by a standard particle-enhanced immunoturbidimetric assay, serum iron measured by a colorimetric assay (ferrozine), and unsaturated iron-binding capacity (UIBC) measured by a spectrophotometric method all using the Roche P Modular system (Roche Diagnostics) and Kamiya Biomedical reagents according to the manufacturer's instructions. Renal function was quantified using estimated glomerular filtration rate (eGFR) in mL/min/1.73 m2 according to the Modified Diet in Renal Disease (MDRD) equation [eGFR = (186.3 × ((serum creatinine1.154) × (age−0.203) × (0.742 if female, otherwise 1) × (1.21 if black, otherwise 1))].
MALDI-TOF MS analysis of hepcidin
The assay was based on the detection and quantification of endogenous hepcidin in plasma relative to that of a stable isotope-labeled hepcidin internal standard using previously described methodologies.25
Inflammatory gene SNP analysis
SNPs in the TNFα (−308G/A, −238G/A), IL-6 (−174G/C), IL-1β (−31T/C, −511T/C, −1464G/C, −3737T/C), leptin (−2548G/A, +19A/G), and leptin receptor (Q223R) genes were evaluated using the TaqMan OpenArray Genotyping System (ABI Biosciences) and imaged on the OpenArray NT Imager as described26 at the Dana-Farber/ Harvard Cancer Center (DF/HCC) High-Throughput Genotyping Core Facility. This system uses 2 allele-specific minor groove binding probes and 2 PCR primers to ensure accurate genotyping.26
Linkage analysis of the leptin −2548 SNP in the Swiss HIV Cohort Study
Because the leptin −2548 SNP (dbSNP ID rs7799039) is not present on the Illumina chips used in previous genome-wide association studies (GWAS) performed in this cohort,27,28 an excellent tagging SNP (rs10487506) located 600 bp away and in perfect linkage disequilibrium (LD) in whites according to Hapmap European (CEU) data, was used as a proxy for the leptin −2548 polymorphism for the analyses. Hemoglobin (Hgb) level was defined as the median of results during the following observation windows: (1) in absence of cART; (2) during the first 6 months of cART; (3) after 6 to 12 months of cART; (4) after 12 to 24 months of cART; and (5) after > 2 years of cART. Linear regression of median hemoglobin was used to test for association between the rs10487506 genotype and Hgb levels, applying an additive genetic model that includes age and the coordinates of 3 significant Eigenstrat axes (to correct for population stratification) as covariates. Considering the substantial difference in study phenotype by gender (mean hemoglobin in nontreated subjects was 13.0 g/dL in females and 14.6 g/dL in males), we ran separate analyses in male and female subpopulations.
Measurement of plasma leptin levels
Leptin levels in plasma were measured using a standard leptin ELISA kit (PeproTech) according to the manufacturer's instructions.
Statistical analysis
For univariate analyses, we used χ2 or Fisher exact test as appropriate for categorical variables. Quantitative variables were compared using the t test. Multivariate analyses were performed with logistic regression (SAS). Correlations were tested using Pearson correlation coefficient.
Results
Factors associated with anemia in VACS
It has been previously established that anemia of any degree is an exceptionally powerful, independent risk factor predicting for poor outcomes in patients with HIV disease.29,30 To gain insight into the pathogenesis of HIV-associated anemia, we analyzed a subgroup of the VACS cohort composed of 2462 subjects with available DNA, blood, and annotated clinical data. Anemia was defined according to WHO criteria (hemoglobin < 13 g/dL and < 12 g/dL in men and women, respectively). Variables of interest stratified by the presence of anemia are shown in Table 1. Of this group, 1597 individuals were HIV+ while 865 were HIV−. A total of 479 subjects were found to be anemic while 1983 were nonanemic. The prevalence of anemia in HIV+ and HIV− subjects was 23.1% and 12.9%, respectively. Independent of HIV status, anemia was present in 23.4% and 8% in blacks and whites, respectively.
Demographic characteristics and variables stratified by anemia in VACS (n = 2462)
| Variable . | Statistic . | All . | Nonanemic . | Anemic . | P . |
|---|---|---|---|---|---|
| Age, y | |||||
| < 40 | n (%) | 299 (12.1) | 257 (13.0) | 42 (8.7) | .001 |
| 41-50 | 1081 (43.9) | 883 (44.6) | 198 (41.1) | ||
| 51-64 | 954 (38.7) | 749 (37.8) | 205 (42.5) | ||
| 65+ | 128 (5.2) | 91 (4.6) | 37 (7.7) | ||
| Sex | |||||
| Male | n (%) | 2332 (94.7) | 1868 (94.3) | 464 (96.3) | .091 |
| Female | 130 (5.3) | 112 (5.7) | 18 (3.7) | ||
| Race | |||||
| White | n (%) | 490 (19.9) | 451 (22.8) | 39 (8.1) | < .001 |
| Black | 1677 (68.1) | 1285 (64.9) | 392 (81.3) | ||
| Hispanic | 203 (8.2) | 171 (8.6) | 32 (6.6) | ||
| Other | 92 (3.7) | 73 (3.7) | 19 (3.9) | ||
| Mortality | |||||
| No | n (%) | 2316 (94.1) | 1882 (95.1) | 434 (90.0) | < .001 |
| Yes | 146 (5.9) | 98 (4.9) | 48 (10.0) | ||
| HIV status | |||||
| Negative | n (%) | 865 (35.1) | 753 (38.0) | 112 (23.2) | < .001 |
| Positive | 1597 (64.9) | 1227 (62.0) | 370 (76.8) | ||
| CD4, n = 1542 | n | 1542 | 1188 | 354 | < .001 |
| Mean (SD) | 421.0 (284.2) | 447.5 (284.9) | 332.3 (263.7) | ||
| Median | 371 | 400 | 287.5 | ||
| (Min, max) | (3.0, 2538) | (3.0, 2538) | (4.0, 1394) | ||
| (25th, 75th) | (221.0, 557.0) | (250.0, 584.5) | (138.0, 439.0) | ||
| HIV-1 viral load, n = 1542 | n | 1542 | 1186 | 356 | < .001 |
| Mean (SD) | 3.1 (1.2) | 3.0 (1.2) | 3.3 (1.3) | ||
| Median | 2.7 | 2.6 | 3 | ||
| (Min, max) | (1.7, 5.9) | (1.7, 5.9) | (1.7, 5.9) | ||
| (25th, 75th) | (1.9, 4.1) | (1.9, 4.0) | (1.9, 4.4) | ||
| Comorbidities | |||||
| None | n (%) | 99 (4.0) | 92 (4.6) | 7 (1.5) | < .001 |
| 1-2 conditions | 951 (38.6) | 827 (41.8) | 124 (25.7) | ||
| > 3 conditions | 1412 (57.4) | 1061 (53.6) | 351 (72.8) | ||
| cART baseline, n = 1597 | |||||
| No | n (%) | 457 (28.6) | 361 (29.4) | 96 (25.9) | .195 |
| Yes | 1140 (71.4) | 866 (70.6) | 274 (74.1) | ||
| BMI, n = 2424 | n | 2424 | 1954 | 470 | < .001 |
| Mean (SD) | 26.8 (5.3) | 27.1 (5.2) | 25.5 (5.3) | ||
| Median | 25.8 | 26.4 | 24.4 | ||
| (Min, max) | (12.6, 50.8) | (12.6, 50.8) | (13.1, 46.2) | ||
| (25th, 75th) | (23.0, 29.7) | (23.6, 30.0) | (22.0, 27.6) | ||
| Ferritin, n = 363 | |||||
| < 10 | n (%) | 2 (0.6) | 2 (0.7) | 0 (0.0) | .639 |
| 10-170 | 161 (44.4) | 130 (43.5) | 31 (48.4) | ||
| > 170 | 200 (55.1) | 167 (55.9) | 33 (51.6) | ||
| Hepcidin, n = 339 | n | 339 | 281 | 58 | .665 |
| Mean (SD) | 22.0 (17.9) | 22.4 (18.9) | 19.5 (11.6) | ||
| Median | 18.1 | 18 | 18.8 | ||
| (Min, max) | (2.3, 125.4) | (2.4, 125.4) | (2.3, 59.3) | ||
| (25th, 75th) | (10.5, 27.3) | (10.7, 28.1) | (9.9, 25.5) |
| Variable . | Statistic . | All . | Nonanemic . | Anemic . | P . |
|---|---|---|---|---|---|
| Age, y | |||||
| < 40 | n (%) | 299 (12.1) | 257 (13.0) | 42 (8.7) | .001 |
| 41-50 | 1081 (43.9) | 883 (44.6) | 198 (41.1) | ||
| 51-64 | 954 (38.7) | 749 (37.8) | 205 (42.5) | ||
| 65+ | 128 (5.2) | 91 (4.6) | 37 (7.7) | ||
| Sex | |||||
| Male | n (%) | 2332 (94.7) | 1868 (94.3) | 464 (96.3) | .091 |
| Female | 130 (5.3) | 112 (5.7) | 18 (3.7) | ||
| Race | |||||
| White | n (%) | 490 (19.9) | 451 (22.8) | 39 (8.1) | < .001 |
| Black | 1677 (68.1) | 1285 (64.9) | 392 (81.3) | ||
| Hispanic | 203 (8.2) | 171 (8.6) | 32 (6.6) | ||
| Other | 92 (3.7) | 73 (3.7) | 19 (3.9) | ||
| Mortality | |||||
| No | n (%) | 2316 (94.1) | 1882 (95.1) | 434 (90.0) | < .001 |
| Yes | 146 (5.9) | 98 (4.9) | 48 (10.0) | ||
| HIV status | |||||
| Negative | n (%) | 865 (35.1) | 753 (38.0) | 112 (23.2) | < .001 |
| Positive | 1597 (64.9) | 1227 (62.0) | 370 (76.8) | ||
| CD4, n = 1542 | n | 1542 | 1188 | 354 | < .001 |
| Mean (SD) | 421.0 (284.2) | 447.5 (284.9) | 332.3 (263.7) | ||
| Median | 371 | 400 | 287.5 | ||
| (Min, max) | (3.0, 2538) | (3.0, 2538) | (4.0, 1394) | ||
| (25th, 75th) | (221.0, 557.0) | (250.0, 584.5) | (138.0, 439.0) | ||
| HIV-1 viral load, n = 1542 | n | 1542 | 1186 | 356 | < .001 |
| Mean (SD) | 3.1 (1.2) | 3.0 (1.2) | 3.3 (1.3) | ||
| Median | 2.7 | 2.6 | 3 | ||
| (Min, max) | (1.7, 5.9) | (1.7, 5.9) | (1.7, 5.9) | ||
| (25th, 75th) | (1.9, 4.1) | (1.9, 4.0) | (1.9, 4.4) | ||
| Comorbidities | |||||
| None | n (%) | 99 (4.0) | 92 (4.6) | 7 (1.5) | < .001 |
| 1-2 conditions | 951 (38.6) | 827 (41.8) | 124 (25.7) | ||
| > 3 conditions | 1412 (57.4) | 1061 (53.6) | 351 (72.8) | ||
| cART baseline, n = 1597 | |||||
| No | n (%) | 457 (28.6) | 361 (29.4) | 96 (25.9) | .195 |
| Yes | 1140 (71.4) | 866 (70.6) | 274 (74.1) | ||
| BMI, n = 2424 | n | 2424 | 1954 | 470 | < .001 |
| Mean (SD) | 26.8 (5.3) | 27.1 (5.2) | 25.5 (5.3) | ||
| Median | 25.8 | 26.4 | 24.4 | ||
| (Min, max) | (12.6, 50.8) | (12.6, 50.8) | (13.1, 46.2) | ||
| (25th, 75th) | (23.0, 29.7) | (23.6, 30.0) | (22.0, 27.6) | ||
| Ferritin, n = 363 | |||||
| < 10 | n (%) | 2 (0.6) | 2 (0.7) | 0 (0.0) | .639 |
| 10-170 | 161 (44.4) | 130 (43.5) | 31 (48.4) | ||
| > 170 | 200 (55.1) | 167 (55.9) | 33 (51.6) | ||
| Hepcidin, n = 339 | n | 339 | 281 | 58 | .665 |
| Mean (SD) | 22.0 (17.9) | 22.4 (18.9) | 19.5 (11.6) | ||
| Median | 18.1 | 18 | 18.8 | ||
| (Min, max) | (2.3, 125.4) | (2.4, 125.4) | (2.3, 59.3) | ||
| (25th, 75th) | (10.5, 27.3) | (10.7, 28.1) | (9.9, 25.5) |
VACS indicates Veterans Aging Cohort Study; cART, combined antiretroviral therapy; and BMI, body mass index.
Given the likely presence of a chronic inflammatory state imparted by infection with HIV, we first examined whether hepcidin may be correlated with Hgb or ferritin in a subgroup of VACS subjects (n = 339). This subgroup consisted of 214 HIV+ and 125 HIV− individuals, with 48 of 56 anemic individuals meeting criteria for AI. Although plasma hepcidin was found to positively correlate with ferritin (r = 0.30, P < .0001, Pearson), we found no correlation between hepcidin and Hgb level in this subgroup (P = .665, Pearson).
A functional polymorphism in the leptin gene promoter is associated with anemia in HIV+ VACS subjects
To examine the impact of chronic inflammation on the development of anemia in HIV+ and HIV− subjects in VACS, we analyzed whether the presence of high-expressing polymorphic alleles in a group of candidate proinflammatory genes with known suppressive effects on erythropoiesis or associated with hepcidin regulation (Table 2) predicted for the development of anemia, and whether that predisposition was linked to HIV status or other clinical or laboratory variables. Univariate analysis of proinflammatory gene SNPs revealed only the leptin −2548 G/A SNP to be significantly associated with anemia (P < .001, χ2). The minor allele of this SNP was present at a frequency of 19% in VACS subjects. Using a multivariate logistic regression model adjusted for age, sex, HIV status, CD4 count, log HIV-1 viral load, body mass index (BMI), and eGFR, we found that the leptin −2548 G/A SNP was independently associated with anemia in HIV+, but not HIV− patients, with the AA and AG genotypes significantly associated with anemia (P < .003 and P < .039, respectively, logistic regression). Because the frequency of the major allele of this SNP is present in greater than 80% of blacks and because non-Hispanic blacks comprise ∼ 68% of the VACS cohort, we could not examine the impact of race in our multivariate model.
Candidate proinflammatory gene SNPs
| No. . | Gene symbol . | Name . | Classic identifier . | dbSNP ID . |
|---|---|---|---|---|
| 1 | IL-6 | Interleukin 6 | −174 G/C | rs1800795 |
| 2 | IL-1B | Interleukin 1β | −31 T/C | rs1143627 |
| 3 | −511 T/C | rs16944 | ||
| 4 | −1464 G/C | rs1143623 | ||
| 5 | −3737 T/C | rs4848306 | ||
| 6 | TNFA | Tumor necrosis factor α | −238 G/A | rs361525 |
| 7 | −308 G/A | rs1800629 | ||
| 8 | LEP | Leptin | −2548 G/A | rs7799039 |
| 9 | +19 A/G | rs2167270 | ||
| 10 | LEPR | Leptin receptor | Q223R | rs1137101 |
| No. . | Gene symbol . | Name . | Classic identifier . | dbSNP ID . |
|---|---|---|---|---|
| 1 | IL-6 | Interleukin 6 | −174 G/C | rs1800795 |
| 2 | IL-1B | Interleukin 1β | −31 T/C | rs1143627 |
| 3 | −511 T/C | rs16944 | ||
| 4 | −1464 G/C | rs1143623 | ||
| 5 | −3737 T/C | rs4848306 | ||
| 6 | TNFA | Tumor necrosis factor α | −238 G/A | rs361525 |
| 7 | −308 G/A | rs1800629 | ||
| 8 | LEP | Leptin | −2548 G/A | rs7799039 |
| 9 | +19 A/G | rs2167270 | ||
| 10 | LEPR | Leptin receptor | Q223R | rs1137101 |
SNP indicates single nucleotide polymorphism.
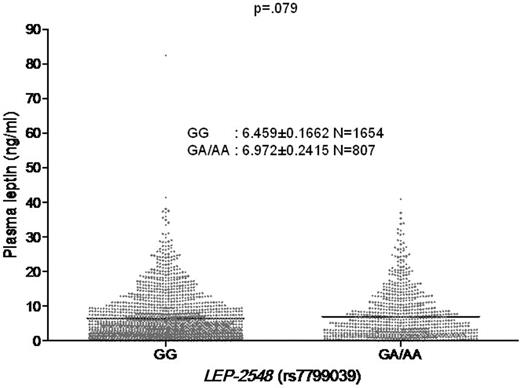
As the AA genotype associated with anemia in our VACS cohort of HIV+ patients has been reported to be associated with higher serum leptin levels in various population studies,13 we next determined whether the AA genotype was associated with higher mean plasma leptin levels in the VACS cohort (n = 2461). Because there were only 132 subjects with the AA genotype, we grouped the AA and AG genotypes and compared leptin levels to the GG genotype. The mean leptin levels were higher in the AA/AG group (6.972 ng/mL) compared with the GG group (6.459 ng/mL). A clustered analysis of AA/AG versus GG genotypes revealed a trend for an association between the AA/AG genotype cluster and higher mean plasma leptin levels that did not meet statistical significance (P = .079, t test; Figure 1).
Association of the leptin −2548 SNP genotypes and plasma leptin levels in the VACS cohort. VACS (n = 2461). Mean plasma leptin levels reflect a single measurement.
Association of the leptin −2548 SNP genotypes and plasma leptin levels in the VACS cohort. VACS (n = 2461). Mean plasma leptin levels reflect a single measurement.
Confirmation of the association between the leptin −2548 SNP and lower Hgb levels in a white HIV+ cohort
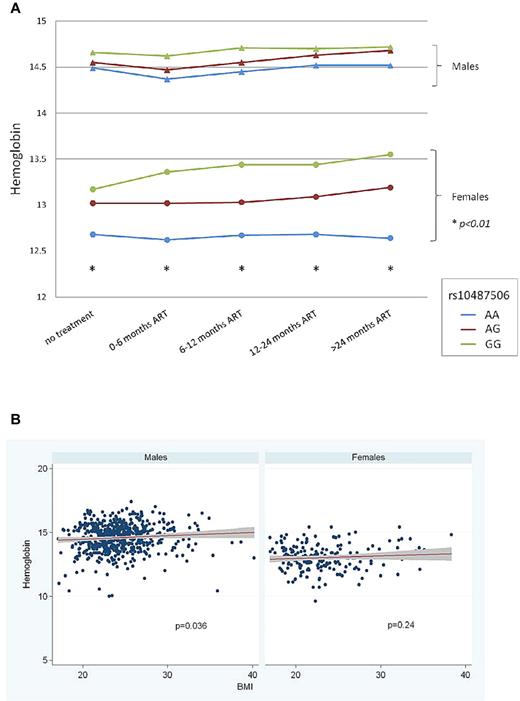
To confirm whether the association of the leptin −2548 SNP and anemia held true in a white population, we next examined this association in the Swiss HIV Cohort Study. Using a tagging SNP (rs10487506) as a proxy for the leptin −2548 SNP (the 2 variants are in perfect LD in whites, Figure 2), we examined the association between leptin genotype and anemia post-cART in 837 HIV+ patients of recent Western European ancestry. The polymorphism was in Hardy-Weinberg equilibrium (P = .7, HWE test), with a minor allele frequency of 43%. Significant associations between rs10487506 and Hgb level were observed in the female subpopulation at all time points: before initiation of ART and repeatedly during treatment (P < .01, linear regression). A trend in the same direction was detectable in males, but did not reach statistical significance (Figure 3A). As some studies have shown that the leptin −2548 G/A SNP correlates with BMI,15 we examined whether there was an association between Hgb and BMI in the Swiss cohort. Although we found that a weak association was indeed present in males, inclusion of BMI as a covariate in linear regression models had no impact on the association between Hgb and rs10487506 (Figure 3B).
Linkage disequilibrium structure of the 5′ region of the leptin gene. Linkage-disequilibrium was calculated using the r2 statistic (r2 = 1) in the HapMap CEU population. Very high linkage-disequilibrium is demonstrated between rs7799039 and rs10487506 in white subjects in the Swiss HIV Cohort (n = 837).
Linkage disequilibrium structure of the 5′ region of the leptin gene. Linkage-disequilibrium was calculated using the r2 statistic (r2 = 1) in the HapMap CEU population. Very high linkage-disequilibrium is demonstrated between rs7799039 and rs10487506 in white subjects in the Swiss HIV Cohort (n = 837).
Association between rs10487506 and hemoglobin in the Swiss HIV Cohort. (A) Significant associations (P < .01) were observed in the female subpopulation at all time points: before initiation of cART and repeatedly during treatment. A trend in the same direction was detectable in males, but did not reach statistical significance. (B) Association of rs10487506 and Hgb stratified by body mass index (BMI). Swiss HIV Cohort (n = 837).
Association between rs10487506 and hemoglobin in the Swiss HIV Cohort. (A) Significant associations (P < .01) were observed in the female subpopulation at all time points: before initiation of cART and repeatedly during treatment. A trend in the same direction was detectable in males, but did not reach statistical significance. (B) Association of rs10487506 and Hgb stratified by body mass index (BMI). Swiss HIV Cohort (n = 837).
Discussion
Our analysis of genetic variability in candidate proinflammatory genes with known suppressive effects on erythropoiesis has resulted in the novel finding that a functional polymorphism in the leptin gene promoter is independently associated with anemia in HIV+, but not HIV−, patients. The pathophysiology of cytopenias in HIV is complex, and may reflect one or several of a combination of abnormalities. Some abnormalities are induced by the HIV virus itself, and others reflect a secondary response to disease-related inflammatory pathways, response to opportunistic infections, or a side effect of cART therapy.31 The chemokine receptors CXCR4 and CCR5 are expressed in hematopoietic progenitors,32 and HIV can directly infect CD34 cells.33,34 HIV infection causes cell death as well as latent infection,35 and may serve as a reservoir of HIV in patients with low or undetectable viral load after cART therapy.36 On the other hand, there are age-related shifts in HSCs that may also predispose to anemia. Studies in mice suggest that aging is associated with cell-intrinsic changes in HSC number and function. HSC numbers actually increase with age, but the cells exhibit functional defects, including decreased homing and altered maturation with a shift toward granulocytic maturation.37,38 Data in humans also show that multipotent progenitor cells increase with age39 and exhibit myeloid skewing.40 Most of these studies focus on the contribution of myeloid skewing to the risk of myeloid malignancy, but because aging is also associated with increased EPO resistance,41,42 it seems likely that the shift toward myeloid proliferation could also be a secondary reflection of poor erythroid maturation. Further studies are required to determine the extent to which anemia in HIV disease reflects latent HIV infection or an accentuation of inflammatory features characteristic of aging.
Analysis of the VACS cohort provides the opportunity to perform studies on the epidemiology and pathogenesis of anemia in a predominantly non-Hispanic black, aging population of veterans both with and without HIV disease. Our data confirm the markedly higher proportion of anemia in non-Hispanic blacks that has been reported elsewhere,43,44 a finding that appears to be independent of HIV status. Given the low prevalence of the minor A allele of the leptin −2548 G/A SNP in blacks and given that the VACS cohort is predominantly composed of non-Hispanic blacks, we could not effectively address whether race or other race-related variables may serve as modifiers of the association between the leptin polymorphism and anemia. However, our confirmation of the association of the leptin −2548 G/A SNP and lower Hgb levels in white HIV+ female patients in the Swiss HIV Cohort Study provides preliminary evidence that this association may be independent of race. It is unclear why we did not find a significant association in male HIV+ subjects in the Swiss Cohort, although reassuringly the trend was in the same direction. It also remains to be determined whether the association of this specific leptin polymorphism and anemia reflects a cell intrinsic defect in HSCs specific to HIV infection or reflects the broader process of physiologic aging, with HIV disease promoting a state of accelerated aging of HSCs. Potentially in support of the latter is our finding that the association of the leptin −2548 G/A SNP and anemia is independent of BMI, consistent with evidence suggesting that the development of leptin resistance appears to be an age-related phenomenon independent of body fat pattern.11 As with all studies examining disease associations, it will be important to confirm these findings in larger and varied series of patients.
Although some studies have linked the leptin −2548 AA genotype with increased leptin levels, we were unable to show a significant association between this SNP and higher plasma leptin levels in the VACS cohort. There is some debate as to whether cytokine levels in the peripheral blood are an accurate measure of chronic inflammatory states.45,46 This is especially true when examining the impact of inflammation on the BM, a specialized compartment where local levels of inflammatory cytokines are almost certainly distinct from levels detected in plasma. Furthermore, cytokine levels may vary and be subject to transient perturbations in acute stresses and comorbidities, rendering single point in time measurements of cytokines difficult to interpret. For these reasons, we feel that genetic variations that determine overall inflammatory responsiveness may be better markers of disease susceptibility than peripheral cytokine levels, particularly with regard to primary disorders of the BM. Alternatively, the leptin −2548 G/A SNP may reflect disordered erythroid maturation stemming either from direct interactions with inflammatory pathways independent of circulating leptin or from indirect inflammatory pathways leading to decreased EPO responsiveness.
Adipokines affect many physiologic processes, but little is known about their impact on hematopoiesis. The increasing recognition of the dynamic role of adipose tissue in cellular pathways makes our identification of the association of the leptin polymorphism and anemia especially exciting. The BM stroma is a site of considerable adipose tissue, and the fat content of the marrow increases with age. Bone marrow adipocytes express high levels of leptin,47 raising the possibility that intramedullary leptin may influence hematopoiesis. Indeed, leptin appears to have direct effects on BM progenitors, stimulating the proliferation of myelocyte over erythroid progenitors even in the presence of excess EPO.18 Leptin may also indirectly modulate erythropoiesis via induction of proinflammatory mediators that suppress erythropoiesis via IL-6/hepcidin-dependent and -independent pathways.21,23,48
In our analysis, we found no association between plasma hepcidin levels and anemia. These findings are consistent with a recent analysis of a subgroup of the InCHIANTI study that examined the association between urinary hepcidin levels, proinflammatory markers, and anemia, and found that while IL-6 and C-reactive protein were associated with anemia and low serum iron, they were not associated with higher urinary hepcidin.49 These findings raise several intriguing possibilities: (1) increased hepcidin may occur only in situations of overt inflammation; (2) a subset of AI may be mediated via hepcidin-independent inflammatory pathways such as TNFα; or (3) single point-in-time measurements of plasma or urine hepcidin may be poor predictors for AI. In addition, leptin likely functions as part of a highly complex adipokine pathway, with leptin and adiponectin appearing to promote cross-regulatory pathways important for the development of cART-related metabolic syndrome and lipodystrophy,50 as well as differential effects on the development of EPO resistance and insulin resistance in hemodialysis patients.19 Therefore, it is reasonable to assume that these distinct adipokines may also have differential effects on erythropoiesis.
In summary, our study offers the novel observation that a leptin gene promoter polymorphism is associated with anemia in distinct groups of patients with HIV disease. We recognize that our study reflects an association between a leptin polymorphism and anemia in HIV+ patients and does not address causality. Furthermore, it remains to be determined whether the association of the leptin −2548 AA/AG genotypes and subtypes of anemia can be validated in HIV− elderly cohorts. Our work supports the premise that anemia is a biomarker of pathogenic inflammatory pathways and that simply treating anemia will likely do little to alter prognosis. Research focused on elucidating inflammatory pathways underlying subtypes of inflammatory anemia is required for the design of efficacious targeted therapeutics. We provide novel insight into biologic processes that may affect the aging HSC in HIV disease and may also provide a foundation for the development of therapies that effectively decrease morbidity and mortality and enhance the quality of life in elderly HIV patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the members of the Swiss HIV Cohort Study (SHCS): J. Barth, M. Battegay, E. Bernasconi, J. Böni, H. C. Bucher, P. Bürgisser, C. Burton-Jeangros, A. Calmy, M. Cavassini, M. Egger, L. Elzi, J. Fehr, M. Flepp, P. Francioli (President of the SHCS), H. Furrer (Chairman of the Clinical and Laboratory Committee), C. A. Fux, M. Gorgievski, H. Günthard (Chairman of the Scientific Board), B. Hasse, H. H. Hirsch, B. Hirschel, I. Hösli, C. Kahlert, L. Kaiser, O. Keiser, C. Kind, T. Klimkait, H. Kovari, B. Ledergerber, G. Martinetti, B. Martinez de Tejada, N. Müller, D. Nadal, G. Pantaleo, A. Rauch, S. Regenass, M. Rickenbach (Head of Data Center), C. Rudin (Chairman of the Mother & Child Substudy), P. Schmid, D. Schultze, F. Schöni-Affolter, J. Schüpbach, R. Speck, P. Taffé, A. Telenti, A. Trkola, P. Vernazza, V. von Wyl, R. Weber, and S. Yerly. They also acknowledge Dr Donald Humphries of MAVERIC for providing VACS samples.
This work was supported by National Institutes of Health grant RO1AG29154 (N.B.).
National Institutes of Health
Authorship
Contribution: G.J.V. designed research, analyzed and interpreted data, and served as first author in writing the manuscript; J.-Y.J. performed experiments and analyzed data; J.T. collected data and performed statistical analyses; H.B. collected data and performed statistical analyses; D.A. performed experiments and analyzed data; H.S. analyzed and interpreted data; M.F. analyzed and interpreted data; K.M. collected data; A.T. collected and analyzed data and performed statistical analyses; J.F. collected and analyzed data and performed statistical analyses; A.C.J. interpreted data and assisted in writing the manuscript; and N.B. oversaw all aspects of the project and designed research, analyzed and interpreted data, and assisted in writing the manuscript.
Conflict-of-interest disclosure: H.S. and D.A. are listed on a pending patent application associated with development of the hepcidin assay used in this study. The remaining authors declare no competing financial interests.
Correspondence: Gary J. Vanasse, MD, Division of Hematology/Medicine, Brigham and Women's Hospital, Harvard Medical School, 75 Francis St, Karp 5216, Boston, MA 02115; e-mail: gvanasse@partners.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal