Abstract
Specific imatinib resistant BCR-ABL1 mutations confer clinical resistance to nilotinib (NIL; Y253H, E255K/V, T315I, F359V/C) and/or dasatinib (DAS; V299L, T315I/A, F317L/I/V/C). Therefore, mutation analysis is recommended for CML patients (pts) after imatinib failure to facilitate selection of appropriate therapy. However, around 40% of chronic phase (CP) pts without these NIL/DAS resistant mutations also fail second line inhibitor therapy. For imatinib resistant pts without these mutations at the time of commencing NIL/DAS therapy (switchover) we investigated whether sensitive mutation analysis could identity pts at risk of poor response to subsequent therapy.
Switchover samples of 220 imatinib resistant pts (DAS n=131, NIL n=89) were analysed by direct sequencing (detection limit 10–20%) and sensitive, high throughput mass spectrometry (mass spec; Sequenom MassARRAY, detection limit 0.05–0.5%), which detects 31 common BCR-ABL1 mutations (approximately 89% of mutations detected in pts receiving imatinib). We previously demonstrated that mass spec could detect NIL/DAS resistant mutations at switchover in an extra 9% of pts compared to sequencing and that these low level resistant mutations were associated with subsequent failure of these inhibitors (Parker et al, JCO. 2011 In Press). Therefore, for the current analysis, pts with NIL/DAS resistant mutations detected by either method (n=45) were excluded since response is already known to be poor in these cases.
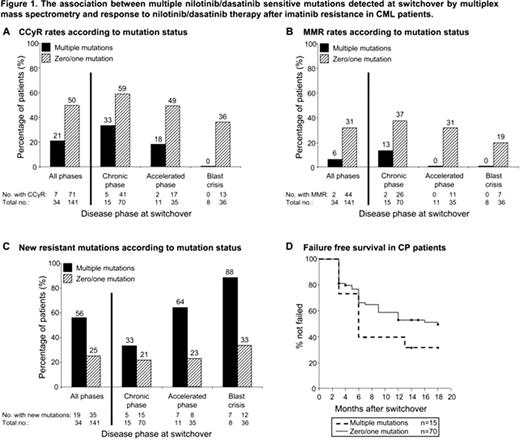
In the switchover samples of the remaining 175 pts, 159 mutations were detected in 86 pts by mass spec, but just 108 mutations were detected in 89 pts by sequencing. Thirteen rare mutations detected by sequencing were not included in the mass spec assay design. Mass spec detected all other mutations detected by sequencing, plus an additional 64 low level mutations. Multiple NIL/DAS sensitive mutations (≥2 mutations) were detected at switchover in more of the 175 pts by mass spec (34/175, 19%; 2–9 mutations per pt) than sequencing (16/175, 9%; 2–3 mutations per pt), P=.009. We divided pts into 2 groups; those with multiple mutations detected by mass spec at switchover (n=34) and those with 0/1 mutation (n=141), and investigated the impact of multiple mutations on response to subsequent NIL/DAS therapy. Pts with 0 or 1 mutation, and similarly pts with 2 or >2 mutations, were grouped together, as no difference in response was observed. The median follow up for CP, accelerated phase and blast crisis pts was 17 (2–33), 18 (1–33) and 3 (1–27) mo, and the frequency of multiple mutations was 18%, 24% and 18%, respectively. During follow up, multiple mutations at switchover was associated with lower rates of complete cytogenetic response (CCyR; 21% vs 50%, P=.003, Fig 1A) and major molecular response (MMR; 6% vs 31%, P=.005, Fig 1B), and a higher incidence of acquiring new NIL/DAS resistant mutations detectable by sequencing (56% vs 25%, P=.0009, Fig 1C). At 18 mo, the failure-free survival rate (European LeukemiaNet recommendations) for CP pts with multiple mutations at switchover was 33% compared to 51% for CP pts with 0 or 1 mutation (P=.26, Fig 1D).
The number of mutations detected per pt by mass spec at switchover (max of 9, 8 of 86 pts with mutations had ≥4, 9%) far exceeded the number concurrently detected by sequencing (max of 3). This suggests that mass spec detected a pool of subclonal mutants, each with a small survival advantage after imatinib therapy that was insufficient for their clonal predominance. Multiple low level mutations may be a marker of an increased propensity for subsequent selection of resistant mutations, possibly driven by genetic instability, demonstrating the advantage of a sensitive multiplex mutation assay.
Hughes:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees. Branford:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Research Funding; Ariad: Research Funding.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal