Abstract
Abstract 1219
Our long-term objective is the development of novel therapies for the safe and effective treatment of hemophilia. In particular a safe and economic prophylactic modality to prevent inhibitors development is therefore highly desirable. To this end we have explored strategies to modulate inhibitor development based on the co-administration of FVIII with other proteins, specifically albumin and IVIG. We hypothesise that such co-administration may modulate immunity to FVIII and result in a safe and cost-effective therapy. Based on some published evidence that FIX and vWF may have a modulatory effect on FVIII, we reasoned that perhaps albumin might also play an immunomodulatory effect on FVIII. We have developed a protocol for infusing recombinant FVIII in hemophilia A mice modelled on the current therapy of hemophiliacs, and in response to this protocol hemophilia mice develop high titre of inhibitors. Thus, this model can be used to assess potential immununomodulatory strategies.
Groups of hemophilia A mice (n=5) were infused weekly IV with 2IU rFVIII for a total of 4 weeks. Three of these groups of mice were also administered human albumin (either 25mg IV, 25mg IP, or 500mg IV) at the same time as FVIII. Mice were bled before the treatment and weekly for the 4 weeks, and total antibodies to FVIII were detected by ELISA. Importantly, while mice receiving FVIII had high antibody titer and were in good health, there was significant mortality in the mice treated with FVIII and albumin. Interestingly, the surviving mice had a very low antibody titre, statistically significant from mice receiving only rFVIII.
Given the mortality, another experiment was designed to test the effect of reduced doses of albumin. Groups of hemophilia A mice (n=5) were infused IV with a weekly injection of 2IU rFVIII for a total of 4 weeks supplemented with either 25μg, 50μg or 100μg of human albumin. Mice were bled at weekly intervals until the end of the experiment (day 28), and the titre of anti-FVIIII antibodies was determined. Most All mice survived the entire treatment and were found in good health. Further, the FVIII antibody titre in the group that received albumin was detectable, but significantly reduced with respect to the mice that received only FVIII. Marked variability among individual mice was observed.
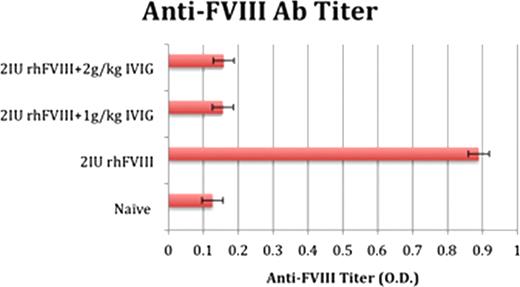
FVIII / IVIG co-administration
Our findings suggest that co-administration of FVIII with albumin and IVIG can modulate the humoral immune response to recombinant human FVIII in hemophilia mice. Further, the observed effect appears to be dependent on the dose of albumin co-administered. It is hypothesized that the observed modulation is due to antigen competition, as previously speculated. Importantly, while IVIG was well tolerated by mice, very serious adverse effects were observed in mice treated with high doses of albumin. Interestingly, even the high dose used represents a small fraction of the physiological levels of albumin in human plasma, which is 3–5 g/dL. IVIG, in contrast, is a known modulator of the immune response in humans, and the study of this modulatory effect in a hemophilia mouse may lead to novel therapeutic strategies, relevant for the management of hemophilia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal