Abstract
Abstract 1481
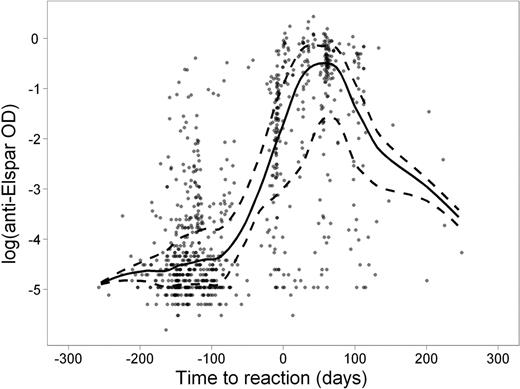
Clinical manifestations of allergy to asparaginase can be subtle, making the diagnosis challenging. The usefulness of antibody tests in differential diagnosis is unclear. We investigated clinical allergic reactions and serum antibodies to native E. coli asparaginase (Elspar) (at 4–5 time points per patient) in 410 children with newly diagnosed acute lymphoblastic leukemia (ALL) treated on St. Jude's Total XV study. Of the 169 patients (41.2%) who exhibited clinical allergy to Elspar, 147 (87.0%) were positive for anti-Elspar antibodies as measured by ELISA. Of the 241 patients who never developed a clinical allergy, 89 (36.9%) had detectable antibodies. Antibody levels were estimated to be at their highest ∼ 50 days after an allergic reaction (Figure). Patients in the low-risk treatment arm were more likely than those in the standard/high-risk arm to have a clinical allergy (51% vs. 32%, P =.0002) and detectable antibodies (69% vs. 47%, P = 6.6 ×10−6) to Elspar. Of those patients positive for antibodies, the antibody exposure (area-under-the-level vs. time curve; AUC) over the entire course of asparaginase therapy (average ± standard deviation) was higher in those who developed allergy (30.5 ± 16.5 optical density × days) than in those who did not (10.8 ± 13.3; P < 1 × 10−15). Serum antibody measurements at week 7 of continuation therapy had a sensitivity of 87–88% and a specificity of 68–69% for predicting subsequent clinical reactions (occurring at weeks 7–9) or for confirming prior clinical reactions (occurring at weeks 1–6 of treatment). The median serum asparaginase activity was lower in patients positive for antibodies (0.05 IU/mL) than in those negative for antibodies (0.2 IU/mL; P = 7.0 × 10−6) 7 days after Elspar administration. After adjusting for age and treatment arm, children with higher antibody AUCs had a lower risk of symptomatic osteonecrosis (odds ratio 0.83; 95% confidence interval, 0.73–0.95; P =.007), a finding which may be caused by higher clearance of dexamethasone due to lower asparaginase activity (Yang et al, J Clin Oncol; 26: 1932–9, 2008). Antibodies to Elspar were significantly related to clinical allergy and were useful as a clinical test to predict or confirm clinical allergy to the drug. Antibodies also were associated with low plasma exposure to active asparaginase, consistent with a lower risk of other adverse effects of ALL therapy.
Anti-Elspar optical density (OD) level in serum samples (dots) relative to the number of days elapsed before (negative) or after (positive) clinical reaction to Elspar. The trend lines of median (solid) and quartiles (dashed) are shown.
Disclosures:
Evans:St. Jude Children's Research Hospital: Employment, Patents & Royalties; Aldagen: Membership on an entity's Board of Directors or advisory committees; NCI, NIH: Research Funding. Relling:St. Jude Children's Research Hospital: Employment, Patents & Royalties; Sigma-Tau Pharmaceuticals, Inc:; NIH: Research Funding.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal