Abstract
Abstract 1854
Human NK cell lines NK-92 and KHYG-1 exhibit cytotoxicity against a broad range of tumor types in vitro, including multiple myeloma (MM). To further test efficacy of the NK lines against MM, we developed a bioluminescent mouse model that recapitulates clinical MM using the human U266 MM cell line transduced to express GFP and luciferase (U266eGFPluc) to monitor disease progression in vivo and assess bone marrow (BM) engraftment.
In a pilot study in which 2×106 U266 cells were injected intravenously into NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (NSG) mice, CD138+ MM cells engrafted in BM, with no detectable engraftment in the liver, lungs, spleen, heart, or kidneys by anti-CD138 immunohistochemistry staining at 10 weeks. We used the U266eGFPluc bioluminescent NSG mouse model to evaluate efficacy of NK-92 cell therapy in vivo. We gave 10×106 NK-92 cells every 5 days to a total dose of 50×106 cells 7 days after MM injection. Tumor burden was monitored weekly by bioluminescence imaging 4 weeks after MM inoculation using the IVIS Imaging System, and LivingImage™ Software was used to acquire images and quantify bioluminescence. We showed that U266eGFPluc cells localized to BM and spine, reflecting MM pathophysiology. Disease burden in the NK-92 treated group was consistently lower than controls over time and significantly lower at 8 weeks (Dorsal and Ventral Mann-Whitney p=0.0381) whereas for KHYG-1, the signal increased slightly over control, but was not significant at 8 weeks (Mann-Whitney Dorsal p=0.540 and Ventral p=0.247). Clinical disease progression in MM control mice correlated with IVIS signal intensity at week 11 (r2=0.4; F test p=0.0279).
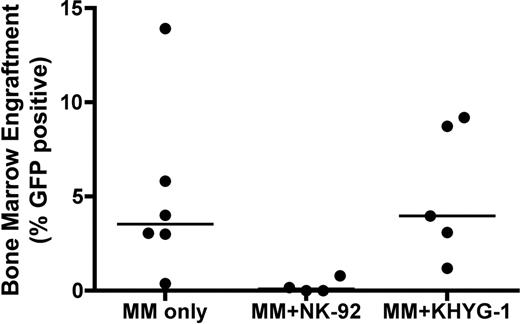
Engraftment was determined by sacrificing mice at 10 weeks and analyzing BM for GFP+ cells by flow cytometry. Engraftment of MM cells in BM was as follows (mean+/− SEM): control (5 +/− 1.9%), NK-92 (0.24 +/− 0.19%) and KHYG-1 (5.2 +/− 1.6%) showing a trend toward a significant decrease in mean engraftment for the NK-92 group versus control (unpaired student's t test p=0.055), but not for KHYG-1 (p=0.939). One of 6 control mice had low engraftment with U266eGFPluc at 10 weeks increasing the variance of the control mean. There was a statistically significant decrease in median engraftment in the NK-92 group (Mann-Whitney p=0.019), but not for KHYG-1 (p =0.792) (Figure). GFP BM engraftment corresponded with bioluminescence detected in R and L BM by IVIS. Presence of NK cells in BM was detected in only 1/3 NK-92 mice tested (0.2%) and in none of the KHYG-1 mice at 10 weeks. To assess biodistribution of KHYG-1 we injected 10×106 CFSE-labeled KHYG-1 via tail vein into healthy NSG mice. Blood and organ samples were collected 8 and 24 hours later and analyzed by flow cytometry. We detected CFSE-labeled KHYG-1 primarily in liver, blood and lung, less in kidney, and none in heart, spleen or BM.
We have established a human MM cell line xenograft model in NSG mice comparable to clinical disease. Treatment efficacy can be monitored in live NSG mice by IVIS imaging technology and tumor burden at sacrifice can be determined by GFP detection. MM progression was reduced by NK-92, but not KHYG-1 as measured by bioluminescence and reduction of engrafted U266eGFPluc cells. We have shown that a MM xenograft model can screen for in vivo efficacy of immune therapies for MM. Our results indicate that NK-92 is a potentially effective therapy for MM.
BM engraftment of GFP+ MM cells at 10 weeks for MM only control mice (left), NK-92 treated MM mice (center) (p=0.019) and KHYG-1 treated MM mice (right) (p =0.772). Line represents median of each sample; each dot represents BM engraftment of 1 mouse.
BM engraftment of GFP+ MM cells at 10 weeks for MM only control mice (left), NK-92 treated MM mice (center) (p=0.019) and KHYG-1 treated MM mice (right) (p =0.772). Line represents median of each sample; each dot represents BM engraftment of 1 mouse.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal