Abstract
Abstract 1858
High dose therapy (HDT) with autologous stem cell transplantation (ASCT) is a standard treatment option for eligible frontline myeloma patients (pts). However, almost all patients ultimately relapse. Thus, new strategies are required to control the residual disease after HDT. Consolidation therapy, given early after HDT, could enhance the depth of response and further improve progression free survival (PFS) and overall survival (OS). We previously reported that either Thalidomide or Lenalidomide given after HDT was able to reduce this residual disease (IFM 99-02 and 2005-02 trials). During last EHA meeting (abstract #510)., Cavo M et al., updated results of their recently published phase 3 trial. Consolidation by Bortezomib-Thalidomide-Dexamethasone (VTD) after VTD induction and double ASCT improved response in 31 % of pts and 61 % of them achieved CR. This translated into a gain of PFS (62 % at 3 years) and a reduction of the relative risk of progression or death of 36 %. The aim of this study was to evaluate the efficacy of early consolidation therapy and its impact on PFS.
In this prospective monocenter study, pts were eligible to receive early consolidation if they had the following: 1) at least partial response (PR) after HD melphalan (HDM), 2) no grade ≥ 2 peripheral neuropathy (PNY). The consolidation regimen was: vTD 61%, Lenalidomide 23%, Lenalidomide plus Dexamethasone 13% and Bortezomib-Lenalidomide-Dexamethasone (VRD) 3%. Consolidation had to be started within 3 months from HDM with no following maintenance. Response was assessed according to International Myeloma Working Group uniform response criteria 1 month after the last cycle of consolidation. The duration of PFS was calculated for all patients from time to HDT to time of progression, relapse, death from any cause or to last contact. PFS was analysed using Kaplan-Meier curves.
From February 2007 to December 2010, 100 frontline MM pts under 65 received HDM followed by ASCT. Seventy six pts were eligible for consolidation (conso group), 24 pts were not (no conso group). After HDT, response rates were: VGPR=29%, and CR=71% in the no conso group, vs PR=20%, VGPR=55%, and CR=25% in the conso group (p<0.001). Early consolidation upgraded response in 17% of pts with PR=11%, VGPR=49%, and CR=36%. Three pts had progressive disease after or during consolidation.
Median follow up is similar for the 2 groups (20 months). Maybe due to unbalanced response repartition, there was no impact of consolidation on PFS (median 25 mos in the 2 groups). Nevertheless, estimated median PFS was not reached in pts achieving CR after consolidation vs 27 mos in pts in CR after HDT in the no conso group (ns).
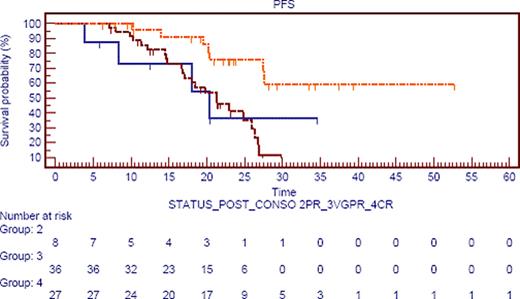
If we focus on response after consolidation, estimated median PFS was not reached in CR pts versus 20 mos in PR pts, and 21 mos in VGPR pts (p=·006; figure 1), irrespective of post HDT status (already in CR or not). Moreover, if we compare pts in VGPR after ASCT (42) we observe two groups: those who upgrade their response to CR (19%) and those who stay in VGPR. The first group achieve the same PFS than patients in CR post ASCT and staying in CR after consolidation (28 mos). Interestingly, those pts (pts upgrading their response) present a longer PFS than those staying in VGPR post consolidation 28 mos versus 20 mos (p=0,032).
Our data suggest that achieving CR after consolidation therapy is a major end-point to improve PFS, maybe even in pts already in CR after HDT. So we could propose to treat pts with consolidation until they obtain CR, they achieve a plateau or they present toxicities.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal