Abstract
Abstract 1918
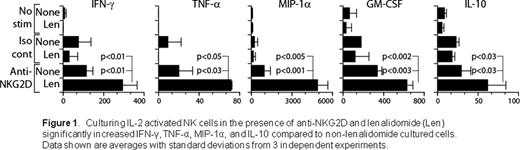
The ability to use natural killer (NK) cells as adoptive immunotherapy to treat malignancies is under intense clinical investigation. Cytokine activation is commonly used to enhance NK cell proliferation (Miller Blood 2005; Laport BBMT 2011). Drawbacks include long-term culture conditions to generate adequate numbers of NK cells and cytokine injections to patients post-infusion to maintain activity. Lenalidomide is an immunomodulatory drug widely used to induce tumor remission in multiple myeloma and myelodysplastic syndrome and has been shown to reduce the incidence of relapse after allogeneic hematopoietic cell transplantation. Lenalidomide has also been reported to augment T and NK cell activation and, coupled with its anti-proliferative and pro-apoptotic activity, could be a novel tool to optimize NK cell adoptive immunotherapy for malignancies. However, the precise molecular mechanisms that are influenced by lenalidomide have yet to be determined. In this study, we analyzed the role of lenalidomide on NKG2D-mediated murine NK cell activation and effector functions. NK cells collected from C57BL/6 mouse spleens were activated with plate-bound anti-NKG2D MAb (A10). After 18 hours, supernatants were collected and assayed using a Bioplex kit to evaluate cytokine and chemokine generation. To assess the ability of NK cells in mediating cytotoxicity through the NKG2D receptor, we tested EL4 murine thymoma cell lines that stably express differing levels of a ligand for NKG2D known as H60 (identified as either H60hi or H60lo depending on level of expression), in 4 hour 51Cr-release assays. Our results show that the NKG2D-mediated production of inflammatory cytokines (IFN-g, GM-CSF, TNF-a, IL-10) and chemokine (MIP-1a) were significantly augmented after the pre-incubation of NK cells for three hours with 5 mM lenalidomide. Specifically, we found at least threefold increases of MIP-1a and TNF-a in lenalidomide-treated IL-2-cultured NK cells compared to non-treated NK cells and twofold increases in IFN-g, GM-CSF, and IL-10 (Fig 1). However, NK cell recognition and cytotoxicity against H60hi- positive tumor cells were unaltered after culturing with lenalidomide (p>0.5). Neither increased drug doses (0.5 to 20 mM) with varied pre-incubation periods (1–18 hours) nor the addition of growth cytokines such as IL-2, IL-12 and IL-18 altered these outcomes. Furthermore, incubating H60hi-positive tumor cells alone with lenalidomide and co-culturing NK cells with targets or antibodies to induce NK cell activation during lenalidomide exposure did not appear to augment NK cell killing (p>0.5). To decipher whether the lack of increased cytotoxicity was a reflection of an already maximized level of NKG2D engagement in H60hi-positive tumor cells, we similarly tested H60lo thymoma cell lines in concurrent experiments and found comparable lack of increased cytotoxicity (p>0.5). To define the mechanism of lenalidomide action, we are currently investigating the phosphorylation status of multiple signaling molecules downstream of the NKG2D receptor in NK cells, with specific focus of its effects on cytokine/chemokine production and its recruitment of other effector populations in both in vitro and in vivo systems. Additionally, we are evaluating other tumor cell lines and fresh tumor samples as targets. Collectively, our in vitro studies demonstrate that lenalidomide-treated NK cells significantly immunomodulate cytokine and chemokine production but have little or no effect on NK cell-mediated thymoma tumor killing. Our preclinical findings may provide insight when designing NK-based adoptive immunotherapy strategies using lenalidomide in the clinical setting.
Disclosures:
Bartlett:Celgene Corporation: Employment.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal