Abstract
Abstract 2099
Redox equilibrium is an important determinant of malaria pathology and host defensive response to malaria parasites. Many anti-malarial drugs are reported to increase oxidative stress in red cells (RBC). Drug-induced hemolysis, particularly in G6PD deficient individuals, limits the utility of approved 8-aminoquinolones such as primaquine. The search for derivatives of primaquine that maintain efficacy without RBC toxicity is hampered by lack of a predictive assay for hemolytic potential. In order to monitor in vivo RBC redox changes in response to anti-malaria drugs, we have established a transgenic mouse line specifically expressing a redox sensitive GFP (roGFP2) in RBC1 . RoGFP2 is an engineered EGFP with 2 cysteines introduced at amino acid positions 147 and 2042 . When oxidized, the 2 cysteines form a disulfide bond, resulting in a protein conformational change that alters the spectral properties of the GFP. By following the ratio of fluorescence emission at 520nm after excitation at 405 vs 488nm, the intracellular redox potential in live cells can be determined. Here we apply this novel mouse model to follow in vivo RBC redox status upon exposure of transgenic mice to a series of 8-AQÕs and control compounds including known hemolytic agents such as dapsone. Unlike in vitro studies, these whole animal experiments incorporate metabolic transformation of inert parent compounds, pharmacokinetics and a time-course that closely models clinical hemolytic reactions in susceptible individuals exposed to the same drugs.
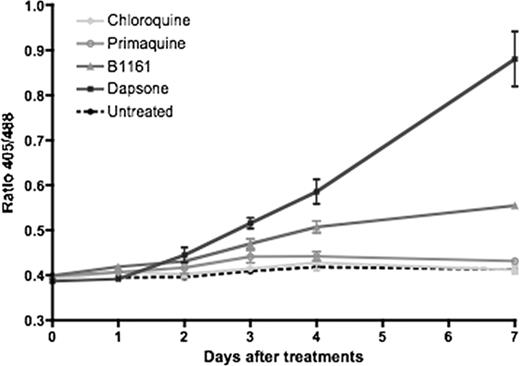
roGFP2 transgenic mice (N=4 per group) were given test compounds (primaquine, chloroquine, dapsone and 1161B*) by gavage 2X/day at a total dose of 50mg/Kg over a period of 5 days. Peripheral RBC were analyzed using a LSRII FACS (BD) to determine the 405/488 ratio, and thus follow the redox status as shown in the figure below. Chloroquine treatment did not cause detectable change in roGFP2 signal, in agreement with previous data that chloroquine does not induce ROS production or hematotoxicity. Treatment with 1161B induced a steady increase in roGFP2 ratio that was first evident at 3 days of treatment, while primaquine treatment has little effect. Dapsone serves as a positive control in this assay, as a known hemolytic agent requiring metabolic activation to produce a redox cycling metabolite.3 Treatment with dapsone produced a time dependent shift to a more oxidized state of roGFP2 that was first evident after 2 days of drug administration. Consistent with these results, CBCÕs obtained one week after start of treatment showed moderate and mild hemolysis in those mice receiving dapsone and 1161B, respectively.
*1161B is the B enantiomer of an 8-aminoquinolone derivative with better efficacy and reduced hematoxicity4,5 in mice, when compared to the racemic mixture of the same compound.
No relevant conflicts of interest to declare.
1.
2.
3.
4.
5.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal