Abstract
Abstract 2191
Integrins are a diverse set of proteins that play a central role in many complex biological processes, such as embryonic development, tumor metastasis, and thrombus formation. The integrin heterodimer is often expressed in a low-affinity, inactive state, relying on specific cytoplasmic or extracellular signals for its activation. One of the major activation pathways that has come to the forefront of integrin research is the membrane-mediated activation of integrin by the cytoskeletal-associated protein talin. While the interaction between talin and integrin is well-established, an atomic-resolution description of the membrane-binding process of talin and of talin-dependent integrin activation has been lacking.
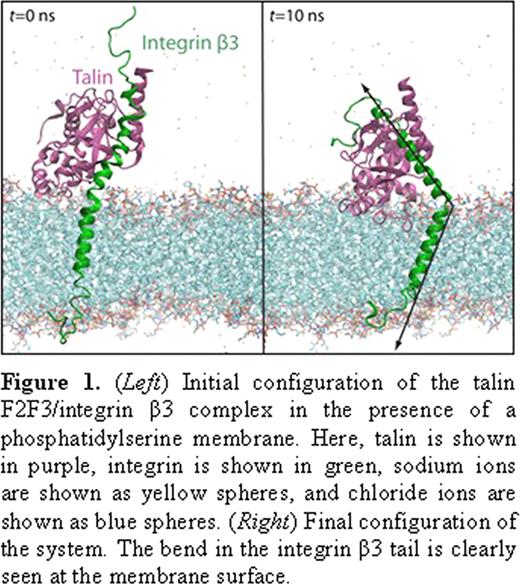
Here, we present a study describing the membrane insertion process of the talin head domain (THD) and its subsequent interaction with the transmembrane domain of integrin αIIbβ3. Using our novel, highly mobile membrane mimetic simulation system, we simulated complete membrane insertion of THD in a phosphatidylserine (PS) membrane a total of six (6) times, revealing key molecular events involved in the process. The THD is initially recruited to the membrane via the documented membrane orientation patch (MOP), consisting of a large number of positively charged residues. However, we also observe a large, interdomain conformational change (> 2.5 nm), which brings the F3 subdomain into contact with the surface of the anionic membrane via residues K325, N326, and K327. Moreover, we characterize a novel, phenylalanine-rich region as the hydrophobic membrane anchor, consisting mainly of F261 and F283, which is released through the snorkeling motion of a few critical lysine residues within the membrane. Although such an anchor has been hypothesized to exist, none had been identified prior to this study.
Finally, the separation of the the α- and β-tails apart wasstudied using slow pulling simulations. These studies have shown that the cytoplasmic ends of the helices interact rather loosely, while the extracellular endsare held together via tight packing of the helices. Free energy profiles of mutants in this region have shown significant decreases in stability of the “off-state”, while mutants in the cytoplasmic end do not show as significant an effect on the free energy landscape of the dimer.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal