Abstract
Abstract 2649
The International Prognostic Index (IPI) is a widely accepted prognostic factor system for diffuse large B cell lymphoma (DLBCL) patients treated with chemotherapy. However, the prognostic value of IPI has been a focal point of the debate in the era of immuno-chemotherapy. Recently, the study of British Columbia group suggested that a revised IPI (R-IPI) which redistributed the IPI factors into 3 distinct prognostic groups provided a more clinically useful prediction of outcome for patients with DLBCL. In order to reassess the value of IPI and R-IPI in unselected Chinese population, we conducted this study.
A multicenter retrospective analysis of DLBCL patients treated with CHOP-like chemotherapy alone or plus rituximab was performed by Shanghai Lymphoma Research Group. In total, 438 patients of newly diagnosed DLBCL treated at 6 participated hospitals were included during the period of 1997–2008. The prognostic value of IPI and R-IPI at diagnosis with regards to overall survival (OS) and progression-free survival (PFS) was evaluated.
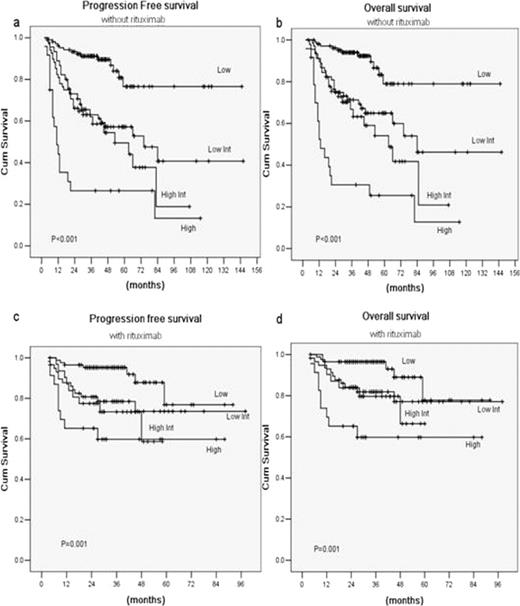
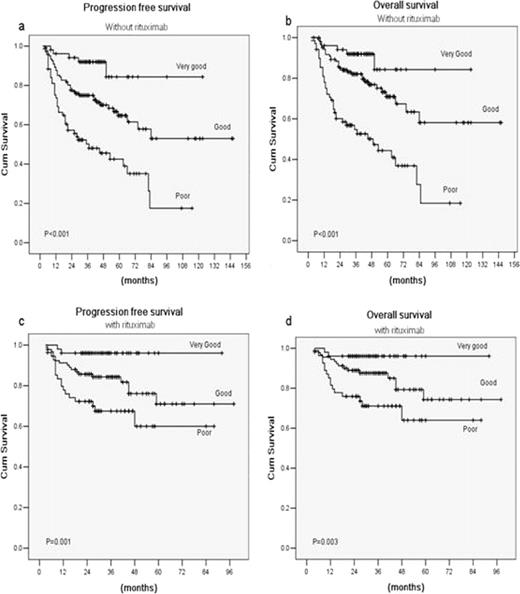
The median age at diagnosis was 50 years (range, 18–83 years), and the median follow-up was 34 months (range, 3–145 months). Among them, 241 patients received CHOP-like regimen, whereas 197 had rituximab (R)-CHOP-like regimen. While IPI remained predictive in CHOP-like group, it could not distinguish between each prognostic category in the R-CHOP-like group (Fig.1). Redistribution of the IPI factors into a R-IPI identified three distinct prognostic groups with significantly different outcomes both in the patients treated with and without rituximab. In R-CHOP-like arm, these three risk groups had distinctly different rates of 3-year progression-free survival rates of 96%, 84.3% and 67.5% (P<0.001), respectively, and 3-year overall survival rates of 96%, 87.6% and 71.1% (P<0.001), respectively (Fig.2).
Outcomes according to the standard International Prognostic Index: Progression free survival (a) and overall survival (b) in the 241 patients treated with chemotherapy alone; Progression-free survival (c) and overall survival (d) in the 197 patients treated with chemotherapy plus rituximab.
Outcomes according to the standard International Prognostic Index: Progression free survival (a) and overall survival (b) in the 241 patients treated with chemotherapy alone; Progression-free survival (c) and overall survival (d) in the 197 patients treated with chemotherapy plus rituximab.
Outcomes according to the revised International Prognostic Index(R-IPI): Progression free survival (a) and overall survival (b) in the 241 patients treated with chemotherapy alone; Progression-free survival (c) and overall survival (d) in the 197 patients treated with chemotherapy plus rituximab.
Outcomes according to the revised International Prognostic Index(R-IPI): Progression free survival (a) and overall survival (b) in the 241 patients treated with chemotherapy alone; Progression-free survival (c) and overall survival (d) in the 197 patients treated with chemotherapy plus rituximab.
Our study underscores the power of R-IPI as a simplified and more clinically relevant predictor of the disease outcomes than the standard IPI in Chinese DLBCL populations in the rituximab era, and it deserves a further study in larger population-based prospective study.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal