Abstract
Abstract 2681
Despite recent advances, the 5 year overall survival for patients with high risk diffuse large B cell lymphoma (DLBCL) is approximately 50% and there is still no known cure for patients with mantle cell lymphoma (MCL). This phase II study of multimodal dose dense therapy evaluated 2 courses of dose intense chemotherapy followed by radioimmunotherapy (RIT) consolidation in patients with previously untreated, mantle cell or high/high intermediate (int) risk aggressive B cell lymphoma.
To evaluate the efficacy and safety of dose intense/dose dense, multimodal chemo-immunotherapy combined with RIT.
Patients with untreated MCL or high int/high risk DLBCL were enrolled. Treatment regimen involved 3 phases of therapy: induction 1, induction 2 and consolidation with RIT (Table 1). Induction 2 occurred approximately 5 weeks after induction 1 and RIT was given 12–24 weeks after rituximab was completed. Patients were evaluated after each treatment phase and those with stable disease (SD) or better and blood count recovery could proceed to the next phase of therapy.
Drug Regimen
| Induction 1 (Ind1): cyclophosphamide (1.5g/m2 daily × 4), etoposide (300mg/m2 q12 hours × 6), rituximab (375mg/m2 weekly × 4) |
| Induction 2 (Ind2): cytarabine (3g/m2 q12 hours × 8), doxorubicin (45mg/m2 daily × 3) |
| Consolidation (Cons): RIT with Iodine 131 tositumomab |
| Induction 1 (Ind1): cyclophosphamide (1.5g/m2 daily × 4), etoposide (300mg/m2 q12 hours × 6), rituximab (375mg/m2 weekly × 4) |
| Induction 2 (Ind2): cytarabine (3g/m2 q12 hours × 8), doxorubicin (45mg/m2 daily × 3) |
| Consolidation (Cons): RIT with Iodine 131 tositumomab |
Thirty nine patients (pts) with high/high int risk DLBCL (n=25) or MCL (n=14) were enrolled. The median age was 60 years (range 21–80).
Common, anticipated toxicities in the induction phases were thrombocytopenia, neutropenia, nausea, fatigue, and anemia. During Ind1 (n=39), grade (gr) III mucositis occurred in 13 pts (33%) and febrile neutropenia (FN) in 31 (79%). Three pts did not proceed to Ind2 due to death (1 candidemia, 1 septic knee prosthesis, 1 from complications of colectomy for prolonged diverticulitis after count recovery) and 2 withdrew to pursue less intense chemotherapy. During Ind2 (n=34) gr III mucositis occurred in 12pts (35%) and FN in 24 (67%). Two pts had gr III/IV cerebellar toxicity that was disabling in 1 pt. Of the 34 pts who received the Ind2, 9 did not receive RIT due to progressive disease (PD) (4), prolonged cytopenias (4), or diagnosis of pancreatic cancer (1). Twenty five pts received RIT and 3 (12%) had FN, 20 (80%) had gr III/IV neutropenia, 23 (92%) had gr III/IV thrombocytopenia, 1 pt died from bacteremia. Two pts developed myelodysplasia 21 and 48 months after starting therapy.
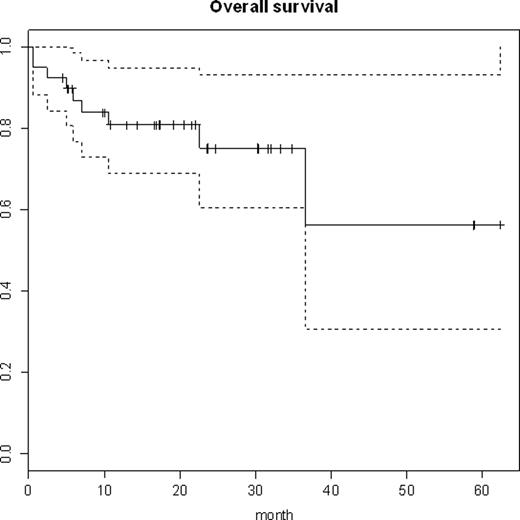
Pts were evaluated for response after Ind1, Ind2 and RIT. 38/39 pts were evaluable for response, with 1 pt withdrawing prior to assessment. The pts who died prior to response evaluation were counted as non-responders. The best overall response rate (ORR) was 95% (36/38) with a complete response rate (CR) of 84% (32/38). See tables 2 and 3 for more detailed response data by phase of treatment and disease type. After a median follow up of 17.2 months, 30 pts (77%) are alive (see figure). The median overall survival for MCL has not been reached and is 36.5 months for DLBCL. Deaths were from Hodgkin lymphoma (1), infection (3), DLBCL (2), complications of surgery (1), MCL (2). The median progression free survival is 36.5 months with 11/14 (79%) MCL and 14/25 (56%) DLBCL pts alive and in continued CR.
Best Response Achieved
| . | ORR . | CR . |
|---|---|---|
| All subjects (n = 38) | 36 (95%) | 32 (84%) |
| DLBCL (n = 24) | 22 (92%) | 20 (83%) |
| MCL (n = 14) | 14 (100%) | 12 (86%) |
| . | ORR . | CR . |
|---|---|---|
| All subjects (n = 38) | 36 (95%) | 32 (84%) |
| DLBCL (n = 24) | 22 (92%) | 20 (83%) |
| MCL (n = 14) | 14 (100%) | 12 (86%) |
Responses after each phase of treatment
| . | ORR . | CR . | PR . | SD . | PD . |
|---|---|---|---|---|---|
| Ind1 (n = 38) | 35 (92%) | 20 (53%) | 15 (39%) | 1 (3%) | 0 |
| Ind2 (n = 34) | 31 (91%) | 29 (85%) | 2 (6%) | 0 | 3 (9%) |
| RIT (n = 25) | 23 (92%) | 23 (92%) | 0 | 0 | 1 (4%) |
| . | ORR . | CR . | PR . | SD . | PD . |
|---|---|---|---|---|---|
| Ind1 (n = 38) | 35 (92%) | 20 (53%) | 15 (39%) | 1 (3%) | 0 |
| Ind2 (n = 34) | 31 (91%) | 29 (85%) | 2 (6%) | 0 | 3 (9%) |
| RIT (n = 25) | 23 (92%) | 23 (92%) | 0 | 0 | 1 (4%) |
The combination of dose dense, dose intense chemotherapy, monoclonal antibody, and RIT demonstrates considerable efficacy, despite expected toxicity, in high risk DLBCL and MCL pts. The response rates seen in this study are higher than expected from standard R-CHOP in this pt population. Further follow up to determine impact on OS and long term complications will be required to confirm these promising outcomes.
Beaven:Glaxo Smith Kline: Family Member Employed by GSK. Off Label Use: Tositumomab is approved for use in relapsed/refractory low grade CD20 positive NHL. It is not FDA approved for first line use in diffuse large B cell lymphoma or mantle cell lymphoma. Neither cytarabine nor etoposide are approved for use in non-Hodgkin lymphoma. Rizzieri:Glaxo Smith Kline: Speakers Bureau. Moore:Glaxo Smith Kline: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal