Abstract
Abstract 2717
Dysregulation of the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signal transduction pathway, which is central to cellular growth, proliferation, metabolism, survival, and angiogenesis, is implicated in Hodgkin lymphoma (HL) pathogenesis. In a previous phase II study of patients with relapsed/refractory lymphomas, the oral mTOR inhibitor everolimus showed promising clinical activity and acceptable toxicity in a subgroup of 19 heavily pretreated patients with HL (Johnston et al. Am J Hematol 2010;85:320–4). The purpose of this study was to confirm the safety and efficacy of everolimus 10 mg/day in patients with relapsed/refractory classical HL.
In this multicenter, US, phase II study, patients aged ≥18 years with classical HL whose disease progressed after high-dose chemotherapy with autologous hematopoietic stem cell transplant (AHSCT) and/or a gemcitabine-, vinorelbine-, or vinblastine-containing regimen were treated with oral everolimus 10 mg/day until disease progression or unacceptable toxicity. Radiological response was assessed every 12 weeks using integrated positron emission tomography/computed tomography with contrast or computed tomography with contrast. The primary endpoint was the overall response rate (ORR) evaluated using modified response criteria for malignant lymphoma (Cheson et al. J Clin Oncol 2007;25:579–86). Secondary endpoints included median progression-free survival (PFS). Adverse events (AEs) were assessed throughout the study and for ≥4 weeks after the last everolimus dose.
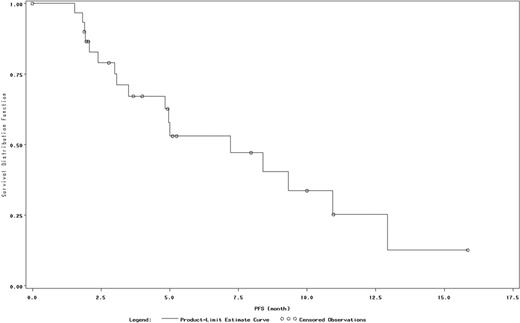
The results from 37 patients in the safety population with evaluable data are reported in this abstract. Among these 37 patients, 35% were male, and the median age was 32 years. The median times from initial diagnosis to the first and most recent recurrence/relapse were 9 and 38 months, respectively. Of the 37 patients, 54% were pretreated with AHSCT and 95% were pretreated with a gemcitabine-, vinorelbine-, or vinblastine-containing regimen. The percentage of patients who experienced disease progression during prior therapies or discontinued their previous treatment due to disease progression was 73%. The median number of prior medication regimens was 4.5. Twenty-two patients discontinued treatment, including 11 due to disease progression and 8 due to AEs. The ORR was 35%, and an additional 27% of patients had stable disease (Table). The median PFS was 7.2 months (Figure). The most common hematologic AEs were thrombocytopenia (38%) and anemia (22%), and the most common nonhematologic AEs were fatigue (43%), cough (27%), headache (22%), dyspnea (22%), and rash (22%). Grade 3 or 4 drug-related AEs were noted in 12 (32%) patients and most commonly included thrombocytopenia (16%), neutropenia (8%), and anemia (8%). Serious AEs were experienced by 19% of patients; no serious AE occurred in more than 1 patient.
Everolimus was associated with a favorable ORR and median PFS duration in highly pretreated patients with relapsed/refractory classical HL, confirming results of a previous study. The AE profile was consistent with that previously observed for everolimus; most AEs were grade 1 or 2 and manageable. These results suggest that further study evaluating everolimus in HL is warranted.
Best Overall Response.
| Best overall response, n (%) . | Everolimus 10 mg/day (N = 37) . |
|---|---|
| ORR | 13 (35.1) |
| Complete response* | 1 (2.7) |
| Partial response | 12 (32.4) |
| Stable disease | 10 (27.0) |
| Progressive disease | 5 (13.5) |
| Unknown | 9 (24.3) |
| Best overall response, n (%) . | Everolimus 10 mg/day (N = 37) . |
|---|---|
| ORR | 13 (35.1) |
| Complete response* | 1 (2.7) |
| Partial response | 12 (32.4) |
| Stable disease | 10 (27.0) |
| Progressive disease | 5 (13.5) |
| Unknown | 9 (24.3) |
Complete response was defined as resolution of all adenopathy.
PFS.
Pinter-Brown:Genentech: Speakers Bureau; Spectrum: Honoraria; Celgene: Consultancy; Merck: Consultancy; Allos: Consultancy. Rogerio:Novartis Pharmaceuticals Corporation: Employment. Warsi:Novartis Pharmaceuticals Corporation: Employment. Graham:Novartis Pharmaceuticals Corporation: Employment. Ramchandren:Seattle Genetics: Research Funding; Novartis: Research Funding; Millennium: Research Funding; Celgene: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal