Abstract
Abstract 3043
In patients undergoing T cell depleted stem cell transplantation (SCT) a high rate of mixed chimerism is seen. Further, chimerism analysis does not consistently predict risks of GVHD or relapse upon withdrawal of immunosuppression (IS) in these patients. Thus, a reliable predictor for the expected evolution of mixed T cell chimerism is needed to help in clinical decision-making for withdrawal of IS and donor lymphocyte infusion (DLI). An alternative immune parameter is T cell recovery post-transplant. We combined this measure with T cell chimerism and examined the predictive value of a calculated donor-derived T cell count for clinical outcomes following SCT.
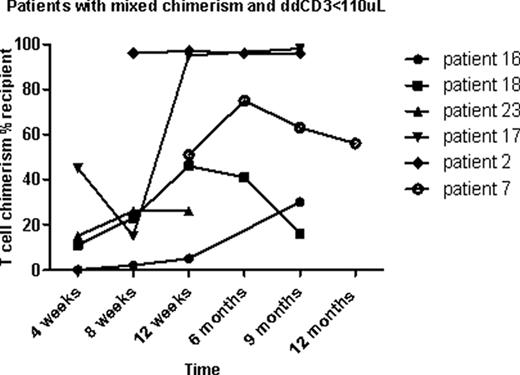
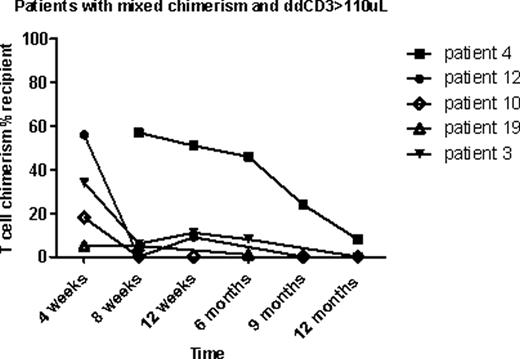
Patients were enrolled in a randomized clinical trial examining two different doses of rabbit anti-thymocyte globulin (ATG 2.5 or 1.7 mg/kg/day; Thymoglobulin®, Genzyme, Cambridge, MA) given IV on day –9 through –7, followed by total body irradiation (4.5 Gray), administered in 3 doses on day –1 and 0 (NCT00709592). Donor engraftment was measured by PCR of short tandem repeat alleles in each donor and patient at 4, 8, 12, and 24 weeks following SCT on whole blood, granulocytes, and total T cells. Between 2008 and 2011, 25 patients were enrolled in this trial, and 22 patients were eligible for this analysis. Nine of the 22 patients had mixed T cell chimerism (≥5% recipient DNA) at 8 weeks post-transplant, and of these 33% (n=3) became fully donor T cell chimeric after withdrawal of IS over the ensuing weeks. One of the 9 patients had improved donor chimerism after DLI. Of the 13 patients who were full donor chimeric in T cells, one patient reverted from full donor to mixed chimerism.
In conclusion, we report the effect of an easily calculable measure of donor T cell reconstitution on clinical outcomes following SCT (particularly GVHD), stability of engraftment and disease relapse. Donor-derived CD3+ cell count may help in the decision-making regarding IS withdrawal and DLI timing in allogeneic SCT recipients. We will be confirming the optimal dd CD3 cell count parameter in larger patient cohorts with comparable disease biology for validation in regimens of varying myeloablative intensities.
Toor:Genzyme: Research Funding. Off Label Use: ATG in stem cell transplantation.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal