Abstract
Abstract 3138
Patient report of symptom burden from Philadelphia chromosome-positive chronic myeloid leukemia (CML) and its treatment is scarce. Symptom burden is the combined impact of symptoms from disease and treatment on daily functioning. Lack of symptom recognition may result in failure to address symptoms and maximize patient functioning and treatment adherence. The purposes of this study are to develop a valid and reliable patient questionnaire for measuring CML symptom burden and to describe symptom burden over 1 year. This abstract reports intermediate results of the longitudinal symptom burden of CML.
160 patients with CML rated the 20 symptom items (13 core cancer and 7 CML-specific symptom items) and 6 interference items of the M. D. Anderson Symptom Inventory for CML (MDASI-CML), on a 0-to-10 scale (0 = not present or no interference; 10 = as bad as can be imagined or complete interference) every 2 weeks using interactive voice response (IVR) telephone technology. Information on performance status, quality of life, disease history, and treatment was collected at study entry. The longitudinal symptom burden of CML and associated factors are reported using descriptive statistics, t-tests, and group-based trajectory and logistic regression modeling.
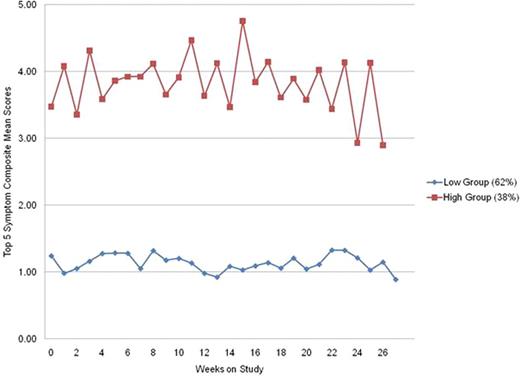
As of May 31, 2011, 160 patients had completed the MDASI-CML for 6 months; 35 patients (22%) had more that 50% data missing and were excluded from analysis. Of the remaining 125 patients, average age at study entry was 50.7 years (standard deviation [SD] = 13.2), 54% were female, 98% had chronic phase (CP) CML, 94% were receiving tyrosine kinase inhibitor (TKI) therapy, average time on current TKI therapy was 44.2 months (SD = 35.7), 2% began TKI therapy at study entry, and 99% had a clinician-rated ECOG performance status of 1 or less. The 5 most severe symptoms reported by the patients were fatigue (mean = 2.73, SD = 2.38), drowsiness (2.17, 2.26), muscle soreness and cramping (2.09, 2.36), disturbed sleep (2.03, 2.37), and difficulty remembering (1.68, 1.88). Trajectory analysis of a composite of the 5 most severe symptoms showed that patients could be divided into 2 groups reporting significantly more (48 patients, 38%) or less (77 patients, 62%) symptom severity across the 6 months of assessment (Figure 1). Average severity of all the individual symptom and interference items as well as the composite means of the top 5 symptoms, the 13 core symptoms, the 7 CML-specific symptoms, and the 6 interference items were significantly different between the groups (all p<0.01). Multivariate logistic regression modeling showed that being unmarried, higher severity of fatigue, difficulty sleeping, difficulty remembering, lack of appetite, easy bruising and bleeding, and lower severity of diarrhea at the time of study entry were independent factors significantly predicting membership in the high symptom severity group (Table 1). No other demographic or disease and treatment variables significantly predicted group membership.
A subset of patients (approximately one third) with CML primarily in CP receiving TKI therapy experiences more severe symptoms and needs special attention to symptom management. A single assessment (the MDASI-CML) can identify patients who experience chronically higher symptom burden and who need interventions to ameliorate symptoms, maintain daily functioning, and encourage treatment adherence. More analysis and research is needed to further delineate the symptom burden of CML and its treatment, understand the effects of symptom burden on treatment adherence, and develop effective interventions to relieve more-severe symptoms.
Factors in CML patients predicting membership in high-symptom severity group.
| . | OR* . | P-Value# . | Lower CI TFU1-3 . | Upper CI TFU1-3 . |
|---|---|---|---|---|
| Unmarried | 13.744 | .017 | 1.585 | 119.154 |
| Fatigue | 1.616 | .002 | 1.184 | 2.204 |
| Disturbed Sleep | 1.676 | .008 | 1.146 | 2.451 |
| Difficulty Remembering | 1.543 | .027 | 1.049 | 2.268 |
| Lack of Appetite | 2.424 | .025 | 1.116 | 5.263 |
| Diarrhea | .594 | .012 | .396 | .890 |
| Easy Bruising/Bleeding | 1.517 | .015 | 1.083 | 2.124 |
| . | OR* . | P-Value# . | Lower CI TFU1-3 . | Upper CI TFU1-3 . |
|---|---|---|---|---|
| Unmarried | 13.744 | .017 | 1.585 | 119.154 |
| Fatigue | 1.616 | .002 | 1.184 | 2.204 |
| Disturbed Sleep | 1.676 | .008 | 1.146 | 2.451 |
| Difficulty Remembering | 1.543 | .027 | 1.049 | 2.268 |
| Lack of Appetite | 2.424 | .025 | 1.116 | 5.263 |
| Diarrhea | .594 | .012 | .396 | .890 |
| Easy Bruising/Bleeding | 1.517 | .015 | 1.083 | 2.124 |
OR = odds ration;
P-value = probability value;
CI = confidence interval
Williams:Novartis: Research Funding. Ault:Novartis: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau. Cortes:Novartis: Consultancy; Novartis: Research Funding; BMS: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal