Abstract
Abstract 3723
Monoclonal antibodies (mAbs) targeting CD20 antigen are now routinely used in the treatment of various types of non-Hodgkin's lymphomas (NHL) and B-cell chronic lymphocytic leukemia (B-CLL). Their antitumor action results from the ability to trigger complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), or immunophagocytosis. Moreover, direct cell death can be induced upon crosslinking with secondary antibodies. Previous studies have demonstrated a sigmoidal correlation between CD20 expression level and rituximab-mediated CDC but not ADCC. Next to CD20 expression level also other potential mechanisms of anti-CD20-mediated cytotoxicity have been observed. Among them an increased expression of complement regulatory proteins (CRP), such as CD46, CD55 or CD59 has been shown to contribute to resistance to mAb-mediated CDC. Antibodies blocking CRP or siRNA that knocks-down CRP expression facilitate rituximab-mediated cytotoxicity. Also in xenograft in vivo models it was shown that antibodies blocking the activity of CD55 and CD59 or a recombinant adenoviral fiber knob protein that cross-links CD46 molecules enhance therapeutic effects of rituximab. Fludarabine, the nucleoside analogue clinically active against CLL and indolent NHL has been shown to act synergistically with rituximab and to down-regulate the membrane expression of CD55 without significantly altering CD20 levels.
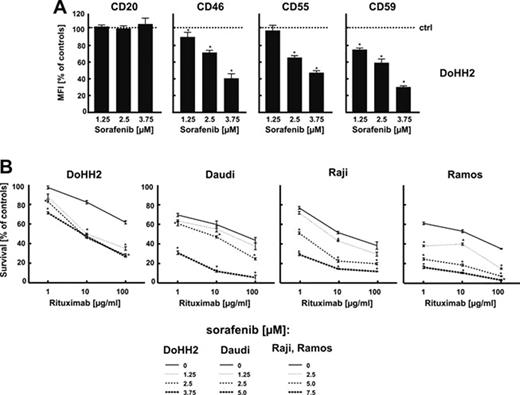
(A) DoHH2 cells were incubated with either diluent or sorafenib at 1.25, 2.5 and 3.75 μM concentration for 48h. Then, cells (1 × 105/100 μl) were stained for CD20, CD46, CD55 and CD59 and analyzed in a FACSCalibur using Cell-Quest Pro software version 5.2. (B) DoHH2, Daudi, Raji or Ramos cells were incubated with either diluent or sorafenib at appropriate concentrations for 48h. Then, equal numbers of cells (1 × 105/well) were incubated for 1h with serial dilutions (from 1 to 100 μg/ml) of rituximab in the presence of 10% human AB serum as a complement source. Cell viability was measured with a MTT assay. The survival of cells is presented as percentage of corresponding diluent- or sorafenib-pretreated cells without rituximab. *P<0.05 (Student's t -test).
(A) DoHH2 cells were incubated with either diluent or sorafenib at 1.25, 2.5 and 3.75 μM concentration for 48h. Then, cells (1 × 105/100 μl) were stained for CD20, CD46, CD55 and CD59 and analyzed in a FACSCalibur using Cell-Quest Pro software version 5.2. (B) DoHH2, Daudi, Raji or Ramos cells were incubated with either diluent or sorafenib at appropriate concentrations for 48h. Then, equal numbers of cells (1 × 105/well) were incubated for 1h with serial dilutions (from 1 to 100 μg/ml) of rituximab in the presence of 10% human AB serum as a complement source. Cell viability was measured with a MTT assay. The survival of cells is presented as percentage of corresponding diluent- or sorafenib-pretreated cells without rituximab. *P<0.05 (Student's t -test).
Altogether, these results indicate that sorafenib, which undergoes clinical trials in patients with NHL and B-CLL might be effectively used in combination with anti-CD20 mAbs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal