Abstract
Abstract 3893
13q deletions (13q-) are the commonest cytogenetic abnormality observed in patients with chronic lymphocytic leukemia as detected by fluorescence in situ hybridization (FISH). It has been observed in 45–55% of patients; in 30% it is the sole cytogenetic abnormality and in this context confers a favourable prognosis. However recent studies highlight that the situation may be more complex, with suggestions that the percentage of interphase nuclei with deletions, and the size of the deletion are key determinants of prognosis. Additionally it remains to be established whether the presence of a homozygous versus a heterozygous 13q deletion plays an additional role. Initial reports suggest that heterozygosity does not impact on time to first treatment or overall survival.
We present the results of 247 patients referred to St BartholomewÕs Hospital, a tertiary referral centre, between 2002 and 2010. Diagnostic specimens were sent including cytogenetic analysis, with a proportion also referred for clinical management. Patients referred only for cytogenetic analysis were excluded from the time to treatment (TTT) and survival data.
Between August 2002 and December 2010, 247 samples were found to have a 13q deletion. Samples included both peripheral blood and bone marrow. Duplicate patient samples were excluded. The probes used were Abbott Molecular IGH@/CCND1 dual colour dual fusion, Abbott Molecular Vysis LSI p53 / LSI ATM and LSI D13S319 / LSI 13q34 / CEP 12 Multi-color or the Cytocell Aquarius CLL Screening Panel.
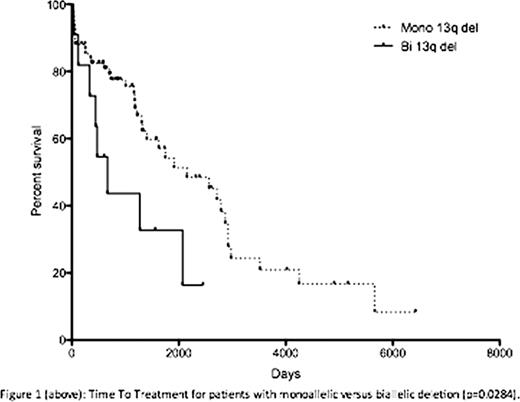
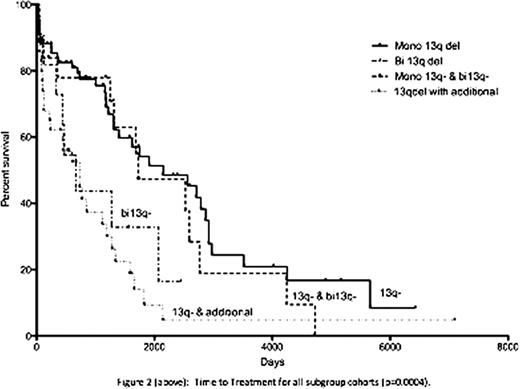
Interphase FISH detected a monoallelic deletion in 133 patients (53.8%) and biallelic in 32 cases (13%). Mosaics of mono- and biallelic deletions within the nuclei were detected in 32 cases (13%). 50 cases (20.2%) demonstrated 13q deletion in addition to other cytogenetic abnormalities. 145 of the 247 patients were managed clinically at St BartholomewÕs Hospital either as the sole centre or in conjunction with their referring hospital. Seventy-eight of the 145 patients had a monoallelic 13q-, in which 38 cases (48.7%) required therapy, with a median TTT of 70.6 months. 12 patients had a sole biallelic deletion. In contrast, of those patients with a biallelic 13q-, 75% required therapy with a significantly shorter TTT (21.9 months, p=0.0284, figure 1). There were 10 deaths within the monoallelic 13q- cohort with a median follow up time of 31 months. For those with bi13q-, three deaths occurred with a median follow up of 17 months. Nineteen patients had a mosaic of monoallelic and biallelic deletions in their nuclei. Thirteen required treatment with a TTT of 56.6 months, with 3 patient deaths. Median follow up was 42 months. Of the remaining 36 patients with additional cytogenetic abnormalities, 28 (78%) required treatment with a TTT of 24 months, in which 15 patients died (median follow up: 16 months). This group was further characterized; 19 patients had an additional 11q22 deletion, which was the commonest abnormality. Nine patients had an additional 17p13 deletion and six had trisomy 12. The remaining 8 cases were either a combined 17p13- and 11q22- or 11q13-.
Two recent studies (171i and 323ii patients) failed to demonstrate a significant difference in TTT and overall survival between patients with a heterozygous and homozygous 13q deletion. In this study, we demonstrated a significant difference in TTT (p=0.0004) amongst the patient subgroups, specifically patients with monoallelic 13q versus biallelic deletions (p=0.0284). Patients with additional cytogenetic abnormalities had the shortest TTT. This group contained patients with 17p deletions that formed 25% of the subgroup. All patient cohorts are currently being characterized further according to age, Rai/Binet stage, CD38 expression, ZAP70 expression, IgH mutational status and percentage of interphase nuclei deleted. As a tertiary referral centre, cases managed at St BartholomewÕs Hospital are skewed towards complex or refractory patients. Whilst patient numbers collated are small, they appear to suggest that a biallelic deletion confers a negative impact on TTT and that 13q deletions may not uniformly represent a good prognostic marker.
No relevant conflicts of interest to declare.
I
II
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal