Abstract
Abstract 426
Single agent arsenic trioxide (ATO) has proven efficacy in the management of newly diagnosed cases of acute promyelocytic leukemia (APL). To validate findings of an initial single center experience (Blood 2006:107; 2627) with this low cost, well tolerated, effective regimen a multicenter study was undertaken in a resource constrained environment. Additionally, in an effort to improve on the earlier experience and study the role of duration of maintenance on reducing late relapses, patients who achieved molecular remission were randomized to 6 vs. 12 months of ATO maintenance.
From July, 2004 to December, 2008, 186 patients were initially screened and enrolled based on morphological diagnosis of APL from 7 centers in India. Twenty seven cases were excluded from analysis (6 RT-PCR negative, 4 IC bleed at diagnosis, 5 septicemia/pneumonia at diagnosis, 9 withdrew consent prior to randomization and some were treated with other protocols, 1 withdrawn by investigator prior to randomization). Patients were treated with single agent ATO at standard doses for up to 60 days in induction; this was followed by 28 day consolidation after a 4 week break following induction. Four weeks after completion of consolidation patients who were in molecular remission were randomized to 6 vs. 12 months of maintenance therapy with ATO administered for 10 days/month. Anthracyclines were permitted in induction for patients presenting with or WBC count rising >20×109/L in the first week, >50×109/L in the second week and for those who developed a differentiation syndrome. Hydroxyurea was permitted for control of leucocytosis. Of the 159 patients who could be evaluated 138 (86.8%) achieved hematological remission (CHR), one patient had primary induction failure and was removed from the study the rest were induction deaths at a median of 17 days (range: 4 – 69). 52 (32.7%) received an anthracycline in induction while 116 (73%) received hydroxyurea in induction. A differentiation syndrome was documented in 25 (16%) cases and was fatal in one. Grade III/IV non hematological toxicity was seen in 26 (16.3%), in the majority they resolved after discontinuing ATO for a short period. A protocol change after randomization was done in 3 cases for persistent toxicity. Five (3.6%) patients did not complete the scheduled maintenance regimen due to poor compliance or was discontinued by the investigator. At a median follow up of 48 months the 5 year Kaplan-Meir estimate of overall survival (OS), event free survival (EFS) and disease free surivival (DFS) was 75.7±3.9%, 68±4% and 76.9±4.2% respectively. Twenty five patients relapsed, the median time to relapse was 19.2 months (range: 8.2–51). There were no relapses beyond 4 years of follow up.
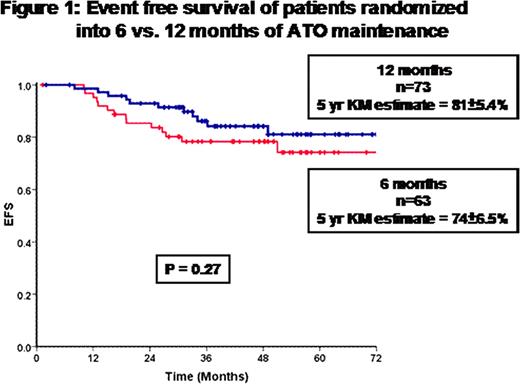
136 patients who achieved molecular remission after consolidation were randomized in to 6 months (n=63) or 12 months (n=73) of maintenance therapy. The baseline characteristics of the two groups were not significantly different. Post randomization, the two groups were analyzed on an intention to treat basis. The OS, EFS (Figure 1) and DFS of the two groups were not statistically different. There was no evidence that the group that received 12 months of maintenance had any increased incidence of toxicity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal