Abstract
Rituximab is widely used in combination with chemotherapy for treating B cell lympho-proliferative disorders. In MCL, two randomised trials explored the addition of Rituximab to standard initial therapy. Neither demonstrated a significant improvement in either PFS or OS. In 2002 the NCRN initiated a phase II randomised trial of FC chemotherapy with or without Rituximab to evaluate response rates. In 2006 this was extended to a phase III study with overall survival as the primary end point.
Newly diagnosed patients with MCL requiring therapy received up to 8 cycles of oral FC (Fludarabine 40mg/m2 and Cyclophosphamide 250mg/m2 both daily × 3) given every 4 weeks with a randomisation to the addition of Rituximab 375mg/m2 on day 1. There was no age limit to the study, no risk stratification and no consolidation of responses with transplantation or maintenance therapy.
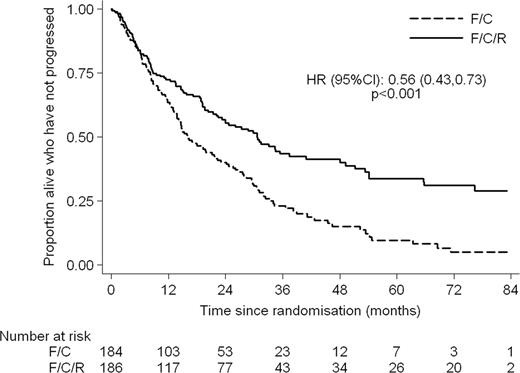
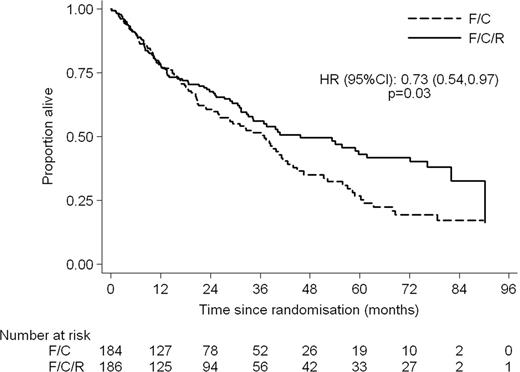
370 patients were randomised. Median age was 66 years (range 36 – 88) with 76% male patients. The arms were well balanced by Mantle Cell International Prognostic Index (MIPI); 77% in the FCR arm and 71% in the FC arm were in the intermediate or high risk groups. 78% and 72% respectively received 4 or more cycles of FCR/FC. At the end of treatment the ORR was 90.6% in the FCR arm and 79.8% in the FC arm (p = 0.01) with CR + CRu rates of 64.7% and 46.9% (p = 0.002) respectively. 5.8% of patients in the FCR arm and 11.9% in the FC arm had PD at the end of treatment. Patients in the FCR arm had longer progression free and overall survival with HRs of 0.56 (95% CI: 0.43–0.73, p < 0.001) and 0.72 (95% CI: 0.54–0.97, p = 0.03) respectively. At a median follow up of 38.8 months the median PFS is 30.6 months in the FCR arm and 16.1 months in the FC arm. The median OS is 45.7 months for FCR and 37 months for FC. The major toxicities were haematological. Significantly more patients in the FCR arm experienced grade 3 or 4 Leucopoenia and Thrombocytopenia however the numbers of grade 4 were not significantly different. Combined grade III/IV toxicity showed 23.3% thrombocytopenia, 45.8% leucopoenia, 12.9% anaemia and 51.4% neutropenia. 11.8% of patients had significant infections. Renal toxicity was modest. 1 patient experienced grade III but there was no grade IV toxicity.
Lymphoma was the commonest cause of death, but 29% of patients in the FCR arm and 24% in the FC arm died of other causes, of which almost half were infection related. An additional 11 patients died of a second malignancy, 4 of whom had AML. 14% of patients in the FCR arm and 10% in the FC arm died without evidence of disease progression.
The addition of Rituximab to FC chemotherapy leads to a significant improvement in both PFS and OS with an acceptable level of additional toxicity. A significant number of patients treated with FC based chemotherapy die whilst in remission of non lymphoma related causes.
Rule:Roche: Consultancy, Research Funding. Off Label Use: Rituximab in mantle cell lymphoma. Follows:Roche: Consultancy, Honoraria. Hillmen:Roche Pharmaceuticals: Honoraria, Research Funding, Speakers Bureau; GlaxoSmithKine: Honoraria, Speakers Bureau; Genzyme: Research Funding; MundiPharma: Honoraria, Speakers Bureau. Walewski:Janssen-Cilag: ; Hoffman La Roche: Honoraria, Institutional/personal grants, travel/accommodation expenses; Mundipharma: Honoraria; Celgene: Honoraria.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal