Abstract
Abstract 4429
Imatinib mesylate (IM) is considered the mainstay of chronic myeloid leukemia (CML) treatment for almost a decade. The primary goal of the study is to share data of a substantial number of CML patients followed at one center.
We analyzed data from 177 CML patients who were treated in our institution and received IM for at least 24 months. They were stratified into low, intermediate and high risk groups based on Sokal score. Early chronic phase (ECP) (within one year from diagnosis to IM start), late chronic phase (LCP) (≥ 12 months from diagnosis), and accelerated phase (AP) CML patients were included in the study. Patients were evaluated for hematologic, cytogenetic and molecular responses, event-free survival (EFS) and overall survival (OS), frequency of adverse events.
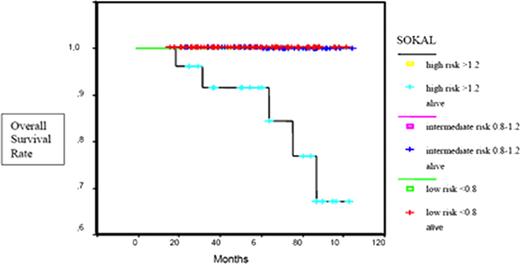
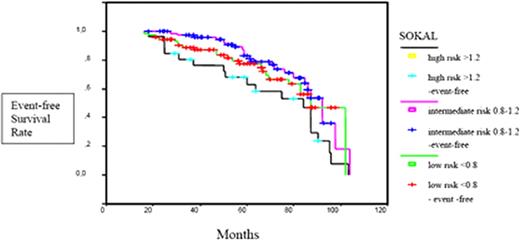
The median age was 51.2 years (range, 22–86 years), with 77 females and 100 males. Patients were followed for a median of 60 months (range, 24–116 months). IM was started at a dose of 400 mg daily. 97.7% were in chronic phase, and 2.3% were in accelerated phase.75.1% of chronic phase CML patients were in early and 24.9% in late chronic phase. 42% of patients were low Sokal risk, 44% intermediate and 14% were high risk patients. 12% of the patients did not receive any prior therapy, 1% had received prior therapy with interferon (IFN), 73% were treated with hydroxyurea (HU) (mostly short course) and 14% with both HU and IFN. Complete hematologic response (CHR) was achieved in 90% of patients at 3 months (median time, 2.02 months). Cumulative rates of cytogenetic and molecular responses at 6, 12, 18 and 24 months are summarized in Table 1. Complete cytogenetic response (CCyR) was achieved in a significantly higher proportion of patients within the low and intermediate Sokal risk group (79.4%, 85.2%) compared with the high risk patients (14.3%, p=0.001). There was a significant difference in the complete molecular response (CMR) ratio achieved by low, intermediate and high Sokal risk patients (70.4%, 63.8% and 33.3%, p<0.05). 5-year OS rates were 100% and 84% among low-intermediate and high Sokal risk patients (p=0.0001) (Figure 1). The EFS at 5 years was 77%, 81%, and 63% in low, intermediate and high Sokal risk patients (p=0.001) (Figure 2). ECP CML patients achieved higher CCyR rates (87%) compared with LCP CML patients (48.6%, p=0.001). CMR rates were 67.7% and 46.9% in ECP and LCP CML patients (p<0.05). 62.2% of the patients remained on 400 mg/day IM treatment and in 3.4% the dose was increased to 600 mg/day. Second-generation tyrosine kinase inhibitors (TKIs) were initiated in 21% of the study population. Hematological and non-hematological toxicities were experienced in 23.7% and 56.7% of our patients. In 6.2% a dose reduction and in 3.4% a switch to a second generation TKI was necessary due to toxicity. 5-year OS and EFS rates of the entire cohort were calculated as 97% and 77%. EFS rates at 5 years were significantly higher among patients achieving CCyR compared with those without a CCyR (92%, 64%, p=0.0001) (Figure 3A). 5-year EFS rates were significantly higher in patients achieving CMR compared with those who did not (95%, 58%, p=0.0001) (Figure 3B).
Cumulative rates of cytogenetic and molecular responses at 6, 12, 18, and 24 months (MCyR=major cytogenetic response; CCyR=complete cytogenetic response; MMR=major molecular response; CMR=complete molecular response).
| Response . | 6 mo (%) . | 12 mo (%) . | 18 mo (%) . | 24 mo (%) . |
|---|---|---|---|---|
| MCyR | 50.3 | 71.8 | 77.4 | 81.4 |
| CCyR | 42.9 | 62.1 | 69.4 | 76.3 |
| MMR | 40.1 | 71 | 73.4 | 80.2 |
| CMR | 34.5 | 48.1 | 60 | 67.3 |
| Response . | 6 mo (%) . | 12 mo (%) . | 18 mo (%) . | 24 mo (%) . |
|---|---|---|---|---|
| MCyR | 50.3 | 71.8 | 77.4 | 81.4 |
| CCyR | 42.9 | 62.1 | 69.4 | 76.3 |
| MMR | 40.1 | 71 | 73.4 | 80.2 |
| CMR | 34.5 | 48.1 | 60 | 67.3 |
Overall survival curves according to the Sokal risk groups.
Overall survival curves according to the Sokal risk groups.
Event-free survival curves according to the Sokal risk groups.
Event-free survival curves according to the Sokal risk groups.
In the current report, we described the outcome of unselected CML patients, treated outside of clinical trials. Grouping patients according to their Sokal prognostic score predicted IM response in this cohort. A longer interval from diagnosis to the start of IM and high Sokal risk score were adverse prognostic factors. 'Real life' data of our study are in accordance with the previous data reflecting the prognostic impact of cytogenetic and molecular responses on survival. Close follow-up of the responses and timely initiation of second-generation tyrosine kinase inhibitors were associated with high survival rates.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal