Abstract
Abstract 4500
Accounting for 4% of childhood hematological malignancy, myelodysplastic syndrome (MDS) is very rare in children than in adults. Therapy of these disorders has been associated with intensive treatment related toxicities and a high risk of relapse. Children with refractory anemia (RA)/refractory anemia with excess blast (RAEB) usually do not respond to acute myeloid leukemia (AML)- type chemotherapy, and chemotherapy without hematopoietic stem cell transplantation (HSCT) resulted in survival rates below 30%. Since failure rates after HSCT are lower in this group when treated at diagnosis, HSCT should be strongly considered, especially when a fully matched sibling donor (MSD) is available. The optimal treatment for children with RA/RAEB without matched sibling donor is presently undetermined. Some of them have stable disease course and require no therapy for years. However, aggressive treatment with HSCT using alternative donors should be considered for severe cytopenia. We analyzed the results of allogeneic HSCT in 16 children who were diagnosed with de novo MDS in a single Korean center. It is the first report about the outcome of HSCT for Pediatric MDS in Korea.
Between November 1997 and June 2011, 16 children with MDS underwent allogeneic HSCT at Asan Medical Center. These patients had RA (n=7), and RAEB (n=9). Peripheral blood smears, bone marrow aspirates and biopsies of all patients were assessed by central morphology review according to the WHO classification. HSCT was recommended if the patients developed neutropenia (ANC<500/μ L) or transfusion dependency. Median age at transplant of 9 males and 7 females was 8.9 years (range 1.6–20.3). Cytogenetic analysis at diagnosis was available in 15 patients. Nine of them had a normal karyotype and 6 patients had miscellaneous chromosomal changes, but nobody had monosomy 7. Six patients (33%) were grafted from an MSD, whereas the remaining were transplanted from an unrelated donor (UD) (n=6), umbilical cord blood (n=3) or a haplo-identical family donor (n=1). All patients received myeloablative conditioning including busulfan (n=13) or total body irradiation (n=3). The median number of transfused CD34 cells was 8.2×106/kg for bone marrow or peripheral blood stem cell transplantation, and 1.3×105/kg for umbilical cord blood transplantation (UCBT). Graft-versus-host disease (GVHD) prophylaxis with cyclosporine and methotrexate was used for 14 patients, and cyclosporine and MMF were used for UCBT.
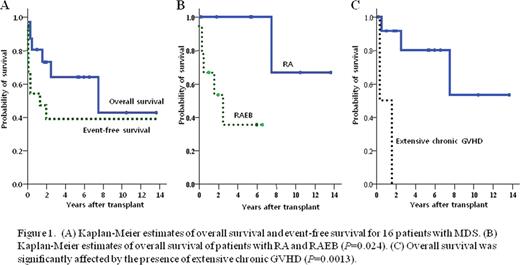
Sustained neutrophil engraftment was achieved in 11 patients, and five patients experienced graft failure. One patient died of subarachnoid hemorrhage on day 1 before engraftment, and 4 patients experienced primary graft failure; all patients were regrafted from haplo-identical related donors. Three patients are alive without disease, but one patient died 103 days after third HSCT from CMV pneumonia. The cumulative incidence of grade II-IV acute GVHD in the 15 evaluable patients was 0.27, and they were all manageable. Two patients developed extensive chronic GVHD; one patient died from disease progression to AML 579 days after HSCT, the other died from fungal pneumonia and intracranial hemorrhage 113 days after HSCT. With a median follow-up of 6.0 years (range 0.1–14), the cumulative incidence of treatment related mortality (TRM) and relapse was 0.19 and 0.20, respectively. The Kaplan-Meier estimates of overall survival (OS) and event free survival (EFS) at 5 years were 0.64 and 0.41. The diagnosis of RAEB was positively associated with relapse (P=0.02) and significantly decreased OS (P=0.024). Donor type was not significantly associated with TRM, graft failure, relapse and outcome. While acute GVHD did not affect the outcome, chronic GVHD significantly affected OS (P=0.0013).
Though our results showed considerable early engraftment failure rates (31.3%), most of all were successfully regrafted form haplo-identical related donors, and showed comparable treatment outcome. Allogeneic HSCT following a myeloablative conditioning can offer a high probability of cure for children with MDS, however, efforts to further reduce the rejection, relapse and TRM in patients undergoing allogeneic HSCT are needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal