Abstract
Abstract 4527
The prognosis for pts with intermediate or high risk AML remains dismal, with relapse rates typically in the 60–80% range after treatment with chemotherapy alone. While allo-SCT can decrease the risk of relapse to 10–20%, widespread use of this modality is limited by relatively high rates of non-relapse mortality (NRM), often due to severe acute and/or chronic GVHD. Attenuation of severe GVHD, without a concomitant increase in relapse or NRM, should improve outcomes and result in cure of a larger fraction of AML pts. We therefore investigated the use of in-vivo T-cell depletion with r-ATG in pts with intermediate- or high-risk AML but without active leukemia at the time of allo-SCT.
Pts (n = 43) were included in this retrospective analysis if they were between 18 and 65 years of age and had no evidence of active AML at the time of allo-SCT (see Table). All pts had 1 or more high-risk features: 1) adverse or intermediate risk cytogenetics (without NPM1 mutation if cytogenetically normal); 2) therapy-related or secondary AML; 3) high WBC count at diagnosis; 4) failure to achieve CR after 1 cycle of induction; or 5) not in CR1 at allo-SCT. Among the 43 pts, 10 received grafts from related donors, 14 from 10/10 matched unrelated donors (URDs), and 19 from mismatched URDs (9/10, n = 11; 8/10, n = 8). All pts received r-ATG according to institutional standard operating policy, with doses ranging from 2.5 – 10 mg/kg depending on donor type and degree of mismatch. All transplants were performed using PBSC. Additional GVHD prophylaxis included tacrolimus plus either methotrexate or mycophenolate mofetil.
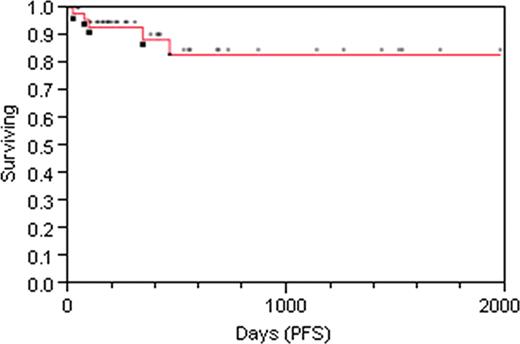
The median age was 47 (range 20 – 65), and median follow-up for surviving pts is 12 (range 1 – 66) months. As of 8/5/11, 39 pts were alive, and 4 had died from multiorgan failure (n = 1), relapse (n = 1), GVHD (n = 1), and veno-occlusive disease (n = 1). The 2-year estimate of PFS is 84.7% (Fig. 1). The 2-year cumulative incidence of relapse is 6.8% (2 pts, days 97 and 147), and of non-relapse mortality 9.4%. Three pts developed severe (grades III-IV) acute GVHD by day 100 (cumulative incidence 4.6% at day 100) with no additional cases of severe acute GVHD beyond day 100. To date, 4 pts have developed moderate/severe chronic GVHD (cumulative incidence 16.8% at 2 yrs), with one death at day 344 related to complications of acute and chronic GVHD. CMV reactivation occurred in 29 pts (56%), with no deaths related to CMV. Three pts have reactivated EBV, with one case of PTLD (all treated with Rituximab).
In this retrospective analysis of single center data, the inclusion of r-ATG in the GVHD prophylactic strategy appeared to significantly attenuate the incidence and severity of both acute and chronic GVHD. Although follow-up is relatively early, the incidence of relapse and NRM does not appear to be increased compared to contemporaneous pts treated without ATG. Given that almost half of the pts received grafts from mismatched URDs, this abrogation in risk of GVHD is significant and clinically relevant. While randomized studies are needed, these data suggest that in-vivo T-cell depletion with r-ATG ameliorates severe GVHD, without increasing relapse or non-relapse mortality, in AML pts without overt leukemia at the time of allo-SCT. Using this strategy, cure rates of 70 – 80% may be realistic and attainable for younger (</= age 65) AML patients who achieve a leukemia-free state and who have a reasonably matched related or unrelated donor.
Patient Characteristics and Outcomes
| Age (median) | 47 |
| >/= 55, n (%) | 19 (44%) |
| Median follow-up (mos.) | 12 |
| AML status at Allo-SCT, n (%) | |
| CR1 | 29 (67%) |
| CR2 | 8 (19%) |
| Blasts <5%, not in CR | 4 (10%) |
| Aplasia | 2 (5%) |
| AML Subtype, n (%) | |
| De-novo | 28 (65%) |
| Therapy-related | 7 (16%) |
| Secondary | 8 (19%) |
| Cytogenetic risk group, n (%) | |
| Good | 4 (9%) |
| Intermediate | 27 (63%) |
| Poor | 12 (28%) |
| Conditioning, n (%) | |
| MA | 21 (49%) |
| RIC | 22 (51%) |
| Donor, n (%) | |
| Related | 10 (23%) |
| URD, matched | 14 (36%) |
| URD, mismatched | 19 (44%) |
| Outcomes | |
| OS, 2yrs | 87.6% |
| PFS, 2 yrs | 84.4% |
| Relapse, 2 yrs | 6.8% |
| NRM, 2 yrs | 9.4% |
| aGVHD III-IV, 100d | 4.6% |
| Severe cGVHD, 2 yrs | 16.8% |
| Age (median) | 47 |
| >/= 55, n (%) | 19 (44%) |
| Median follow-up (mos.) | 12 |
| AML status at Allo-SCT, n (%) | |
| CR1 | 29 (67%) |
| CR2 | 8 (19%) |
| Blasts <5%, not in CR | 4 (10%) |
| Aplasia | 2 (5%) |
| AML Subtype, n (%) | |
| De-novo | 28 (65%) |
| Therapy-related | 7 (16%) |
| Secondary | 8 (19%) |
| Cytogenetic risk group, n (%) | |
| Good | 4 (9%) |
| Intermediate | 27 (63%) |
| Poor | 12 (28%) |
| Conditioning, n (%) | |
| MA | 21 (49%) |
| RIC | 22 (51%) |
| Donor, n (%) | |
| Related | 10 (23%) |
| URD, matched | 14 (36%) |
| URD, mismatched | 19 (44%) |
| Outcomes | |
| OS, 2yrs | 87.6% |
| PFS, 2 yrs | 84.4% |
| Relapse, 2 yrs | 6.8% |
| NRM, 2 yrs | 9.4% |
| aGVHD III-IV, 100d | 4.6% |
| Severe cGVHD, 2 yrs | 16.8% |
Reeder:Celgene: Research Funding; Millennium Pharmaceuticals Inc.: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal