Abstract
Abstract 4919
Hermansky Pudlak Syndrome (HPS) is a genetically heterogeneous group of rare disorders that are classified as ‘lysosome biogenesis disorders’ and are characterized by variegated pigmentation, variable immune deficiencies, and platelet dysfunction. There are 8 known variants of HPS in humans. Lysosomes are membrane-bound organelles that contain hydrolytic enzymes necessary for intracellular degradation and represent the main cellular degradation compartment within the vacuolar system of the eukaryotic cell 1.
Biogenesis of lysosomes partly depends on the function of the biogenesis of lysosome-related organelles complex-1 (BLOC-1), −2, and −3 proteins2. The mutated proteins known to give rise to the HPS variants occur in one or more of the cytosolic BLOC complexes, each being composed of different proteins, including the Adaptor Proteins 1–4 (AP1-4) 3. Also, since mutations in the AP3B1 gene encoding the b subunit of the AP3 complex cause the only HPS subtype which is associated with an immune disorder along with other lysosomal abnormalities 4, this indicates that AP3 is important for lysosome function within the immune system. In the absence of AP3, cytotoxic T lymphocyte (CTL) mediated killing is severely impaired and cytotoxic lysosomal granules are unable to move along microtubules when the CTL recognizes target cells 5.
We report a case of Hermansky Pudlak Syndrome Type 2 in a consanguineous female infant of Arabic origin who presented with severe sepsis, occulocutaneous albinism, platelet nucleotide deficiency, neutropaenia and a homozygous balanced inversion of chromosome 5; inv(5)(p15q13). Further molecular genetic analysis revealed a breakpoint in the AP3B1 gene, thereby confirming a genetic diagnosis of HPS2.
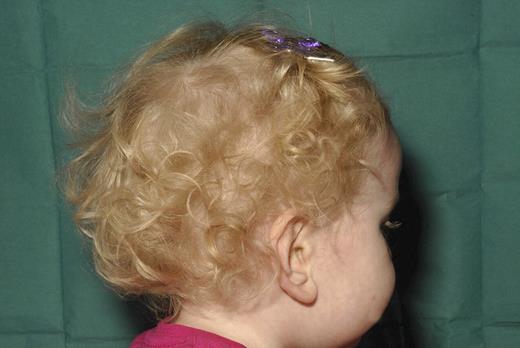
A five month old female infant presented with respiratory sepsis requiring ventilation. Clinical examination revealed occulocutaneous albinism (Figure 1) and hepatosplenomegaly. Laboratory data revealed severe neutropenia (0.2×109/l), CD4 lymphopaenia (446/microlitre), normal immunoglobulins, marked reduction of platelet nucleotides and abnormal platelet aggregation studies.
Photographs showing occulocutaneous albinism in proband.
There was no evidence of haemophagocytic syndrome at presentation. Neutrophil activity was normal and there was no evidence of B-cell dysfunction. Unexpectedly, her CTLs demonstrated normal lysosomal granule release on activation.
Karyotyping showed pericentric balanced inversion in chromosome 5, inv (5)(p15q13). Parental karyotyping revealed heterozygous inversion of chromosome 5 at the same breakpoint. Array comparative genomic hybridization confirmed absence of copy number gain or loss in the inversion. Bacterial artificial chromosome cloning using Fluorescent in situ hybridization confirmed the breakpoint to be within the APB3B1 gene.
She made a good recovery from her initial sepsis and continues to remain neutropaenic and CD4 lymphopaenic on follow up for 12 months, and has required a number of admissions with viral sepsis (adenovirus and respiratory syncitial virus).
In summary, this is a first report of homozygous pericentric balanced inversion of chromosome 5 inv(5)(p15q13), leading to disruption of the AP3B1 gene, giving rise to a clinical phenotype of Hermansky Pudlak Syndrome Type 2. The normal cytotoxic lymphocyte function remains unexplained. Further molecular genetic work is in progress to map the breakpoint in the AP3B1 gene and the corresponding gene(s) involved in the inversion.
No relevant conflicts of interest to declare.
1.
2.
3.
4.
5.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal