Abstract
Abstract 4975
Brentuximab vedotin (SGN-35) is an investigational CD30-directed antibody–drug conjugate consisting of the monoclonal chimeric antibody cAC10 specific for human CD30, the potent antimicrotubule agent monomethyl auristatin E (MMAE), and a protease-cleavable linker that covalently attaches cAC10 to MMAE. Two single-arm pivotal phase 2 trials in relapsed or refractory Hodgkin lymphoma (HL; SG035-0003, Younes et al, ICML 2011) and systemic anaplastic large-cell lymphoma (sALCL; SG035-0004, Shustov et al, ICML 2011) have shown brentuximab vedotin (1.8 mg/kg IV every 3 weeks for up to 16 cycles) to have durable antitumor activity in terms of overall response rate and complete remission (CR) rate with a manageable safety profile. In the absence of a suitable comparator regimen for the conduct of these trials, and per the ICH E10 (CHMP/ICH/364/96) guideline, we conducted meta-analyses of historical chemotherapy data to compare the activity of brentuximab vedotin in the pivotal single-arm trials to the activity of other therapies used for the treatment of relapsed or refractory HL and sALCL.
CR rate was the endpoint since in both HL and aggressive non-Hodgkin's lymphoma (NHL), achievement of CR has been shown to correlate with long-term clinical benefit (Josting & Schmitz, 2004; Lee et al, Ann Oncol 2011). For the HL meta-analysis, a PubMed search identified all prospective trials and retrospective case series in which ≥10 adult HL patients with relapsed or refractory disease were treated with gemcitabine alone or in combination; these regimens were selected for the control population because they constitute one of the most commonly used systemic therapies beyond second-line treatment in HL. Trials were classified as post-autologous stem cell transplant (ASCT; ≥33% of enrolled patients), or ASCT-naïve. For the sALCL meta-analysis, a PubMed search identified all trials since 2001 of aggressive relapsed or refractory NHL that included at least one sALCL patient. Due to the rarity of relapsed or refractory sALCL and the absence of standard therapy in this setting, no specific regimen was selected as a control. Quantitative statistical meta-analyses were carried out per pre-specified plans to minimize bias and ensure integrity of the statistical analysis, and performed using random effect models; estimates (95% CI) of overall CR rate were provided.
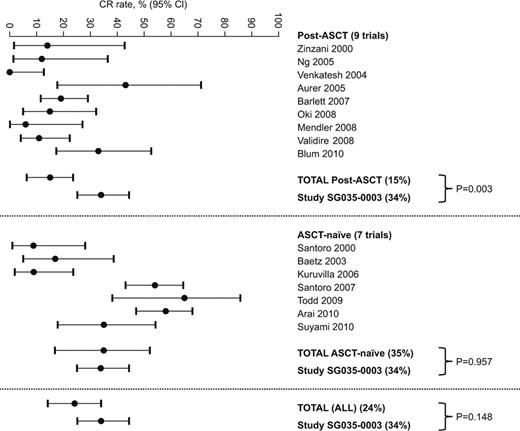
For the HL meta-analysis, 16 trials with 605 patients were identified, including nine post-ASCT trials (n=296, median of three prior regimens) in which a median of 44% (range 33–64%) of patients had prior ASCT, and seven ASCT-naïve trials (n=309, median of one prior regimen). CR rates are shown in the figure. The estimated overall CR rate in the post-ASCT subgroup was 15% (95% CI: 6.5, 23.5), which was significantly lower than the CR rate of 34% (95% CI: 25.2, 44.4; p=0.003) seen with brentuximab vedotin in SG035-0003, in which all patients had prior ASCT and the median number of prior regimens was 3.5. In the ASCT-naïve subgroup, the estimated overall CR rate was 35% (95% CI: 16.9, 52.2), which was not significantly different versus SG035-0003. However, patients in the historical ASCT-naïve subgroup had less advanced disease and were less heavily pretreated. Considering all trials, the estimated overall CR rate was 24% (95% CI: 14.2, 33.9), with a trend towards lower activity versus SG035-0003 (p=0.148). For the sALCL meta-analysis, 19 trials with 817 NHL patients (median of two prior regimens) were identified, which included 1.6–20.8% sALCL patients (n=48 in total). The estimated overall CR rate of 18% (95% CI: 11.3, 24.5) was significantly lower than with brentuximab vedotin in SG035-0004 (53% [95% CI: 39.9, 66.7], p<0.0001).
The anti-tumor activity of brentuximab vedotin in relapsed or refractory post-ASCT HL patients, in terms of CR rate, appears to exceed that of gemcitabine-based therapies in this setting, and appears comparable to that seen with combination chemotherapy in ASCT-naïve patients with less advanced disease. The anti-tumor activity of brentuximab vedotin also appears to exceed that of other treatment options for aggressive relapsed or refractory NHL, including sALCL. Acknowledging the limitations of these comparisons, these meta-analyses suggest that brentuximab vedotin may provide meaningful long-term clinical benefit in relapsed or refractory HL and sALCL.
Gualberto:Millennium Pharmaceuticals, Inc.: Employment. Off Label Use: Brentuximab vedotin for the treatment of relapsed or refractory Hodgkin lymphoma and systemic anaplastic large-cell lymphoma. Chi:Millennium Pharmaceuticals, Inc.: Employment. Liu:Millennium Pharmaceuticals, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal