Abstract
Abstract 719
Platelets play an important role in the maintenance of hemostasis in mammals. Due to the short shelf life of 5 days, availability of transfuseable platelets is limited, especially in cases of traumatic injuries in rural or isolated areas and combat. Clearly, an unmet medical need for a stable and efficacious hemostatic agent exists. In this presentation we describe the development of a safe and efficacious lyophilized platelet derived hemostatic product (LPDH) now under review (IND) by the FDA.
LPDH were manufactured by lyophilization using human in date stored platelets (hIDSP) as a source material and a proprietary process developed by Cellphire Inc. Upon product rehydration, increased number of microparticles (size <1.03 μm; 35.1± 7.5 VS 14.6± 6.6%) and macroparticles (>5.04 μm; 0.74± 1.2 VS 0.02± 0.02%) and decrease in platelet sized particles (>1.03 to 5.05; 56.2± 7.4 VS 88.6 ± 6.7%) were observed as compared to the starting platelet suspension as measured by Beckman Coulter Multisizer™3. Rehydrated LPDH exhibited an increase in Annexin V binding (85.5±10.9 VS 3.8±1.4 % in fresh platelets) in conjunction with a decrease in GPIb expression (from 97.99±2.36 to 44.36±21.7%).
Suspension of LPDH in plasma revealed significantly attenuated aggregation in response to standard agonists (ADP, AA, collagen and ristocetin) as compared to the starting platelet pool. However, LPDH suspensions in buffer demonstrated preserved responses of thrombin-induced aggregation (78±10 VS 95±1.5% in platelets). In plasma LPDH produced thrombin in a concentration dependent manner as indicated by the rate and extent of total thrombin generation by concentrations between 50,000-500,000 particles/μl. Notably the “lag time” of thrombin generation was also significantly shortened.
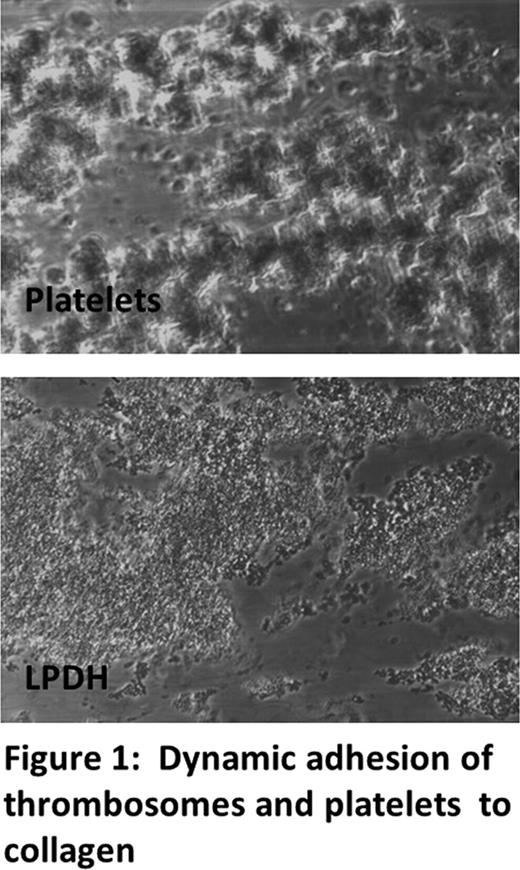
The ability of LPDH to enhance clot formation and stabilization was examined by the Thromboelastography method. At equivalent platelet concentration of 200k/μl, LPDH generate fibrin at 80% rate as compared to platelets, resulting in clot strength ∼85% to that generated by 200,000/μl of hIDSP. LPDH retained the innate ability to adhere to glass-immobilized collagen under high shear similar to freshly isolated platelets. The apparent difference in light diffraction between platelets and LPDH-collagen mediated adhesion assembly remains to be studied (fig 1). 111In labeled LPDH were used to monitor pharmacokinetics and biodistribution in rabbits. 30% to 40% of the LPDH remained in the systemic circulation 5min after infusion (T½ a) and persisted with T½ b of 20h (15 - 20%). 111 In labeled LPDH were found to accumulate primarily in liver (∼58%), and spleen (∼1.3%) with minor accumulation in kidney, lung, heart, muscle, bone and skin. Fifteen percent of the LPDH dose remained in circulation at 24hr.
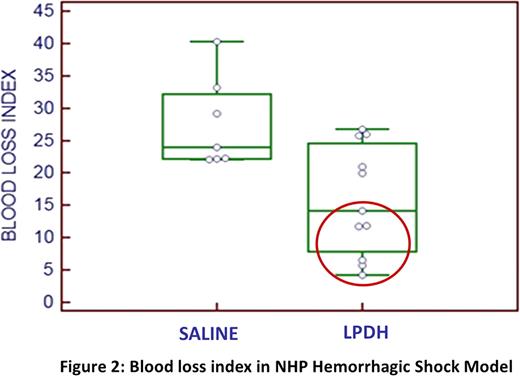
In vivo efficacy was evaluated in a “Thrombocytopenic rabbit ear bleed model” and a “NHP hemorrhagic shock model”. Infusion of LPDH at 1–3% of normal NZWR total circulating platelet count (TCPC) resulted in a significant reduction of blood loss (<0.02) as compared to saline treated NZWR. IV administration (10min) of LPDH at 2–20% of the NHP TCPC resulted in 57% reduction in the blood loss index as compared to saline treated controls (fig 2).
Safety studies conducted under GLP using high and low doses of LPDH in both canine and rabbit, showed no evidence of either macroscopic or microscopic clots or hemorrhages. Hematological and hemostasis biomarkers remained within normal ranges, and were not significantly different from control groups in the rabbit or dog studies.
Fitzpatrick:Cellphire Inc.: Employment, Equity Ownership. Dee:Cellphire: Employment. Koh:Cellphire Inc.: Employment. Cliff:Cellphire: Employment, Equity Ownership. Feurestein:Cellphire: Consultancy. Tandon:Cellphire Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal