Abstract
Abstract 795 FN2
FN2
Ruxolitinib (INC424), a potent and selective oral JAK1 and JAK2 inhibitor, demonstrated rapid and durable reductions in splenomegaly and improved disease-related symptoms, role functioning, and quality of life (QoL) in 2 phase 3 studies in patients with myelofibrosis (MF) (the COMFORT studies). Per-protocol, patient-reported, health-related QoL (HRQoL) and symptoms analyses in the COMFORT-II study are limited. Here we report on additional post hoc analyses of these outcomes.
The COMFORT-II study includes 219 patients (ruxolitinib, n=146; best available therapy [BAT], n=73). At entry, all patients were classified into intermediate 2-risk or high-risk prognostic groups (Cervantes F, et al. Blood, 2009;113(13):2895-2901) and had palpable splenomegaly ≥5 cm below the costal margin. Patients could have received prior therapy for MF. On BAT, doses and schedules or no therapy were selected by the investigator; therapy adjustments were permitted during the randomized treatment phase at the investigator's discretion. Patients in both arms continued in the randomized treatment phase as long as there was no protocol-defined disease progression.
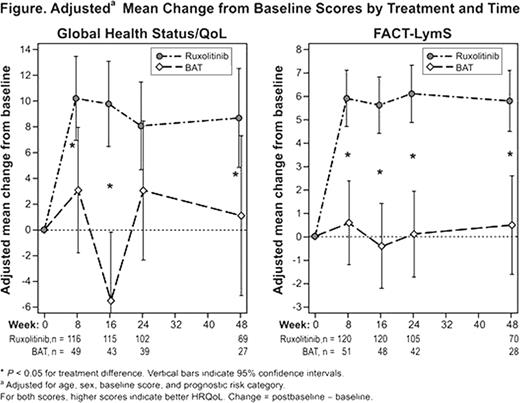
The European Organisation for the Research and Treatment of Cancer (EORTC) QoL Questionnaire–Core 30 (QLQ-C30) and Functional Assessment of Cancer Therapy–Lymphoma (FACT-Lym) questionnaires were assessed at baseline and weeks 8, 16, 24, and 48. EORTC QLQ-C30 consists of 30 items combined into 15 subscales (Global Health Status/QoL, 5 functioning subscales, and 9 symptom subscales; scores range from 0 to 100, and higher scores indicate better HRQoL and functioning but a worsening of symptoms). FACT-Lym consists of 42 items combined into 8 subscales (4 well-being subscales; 1 symptom subscale [LymS]; and 3 total scale scores: FACT-General [G], FACT-Lym trial outcome index [TOI], and FACT-Lym total; scores for the different subscales vary from 0–28 to 0–168, and higher scores indicate better outcomes).
This analysis includes evaluable patients in the randomized treatment phase and assesses changes from baseline in HRQoL and MF symptom scores, including the EORTC subscales, LymS, and FACT total scores, which incorporate well-being and/or symptom subscales. Mixed-model analyses, adjusted for age, sex, baseline score, and prognostic risk category, are used to evaluate treatment differences at each time point and overall across time.
HRQoL and MF symptoms, on average, improved compared with baseline for patients receiving ruxolitinib, but remained the same or worsened for patients receiving BAT.
Based on the EORTC QLQ-C30, the treatment differences in physical functioning, role functioning, fatigue, and appetite loss significantly favored ruxolitinib starting at week 8 (P <.05) and remained significant at week 48 (P <.05). The overall between-treatment differences (on average across time) in adjusted mean change from baseline for MF symptom scores (95% confidence interval) were: Fatigue, –10.2 (–15.9, –4.5; P <.001); Dyspnea, –11.6 (–17.6, –5.6; P <.001); Appetite loss, –16.3 (–21.5, –11.1; P <.0001); Insomnia, –9.8 (–16.7, –3.0; P <.01); and Pain, –9.0 (–14.9, –3.0), P <.01); negative values favor ruxolitinib. Global Health Status/QoL (Figure) was significantly improved in the ruxolitinib arm compared with the BAT arm at weeks 8, 16, and 48.
Scores on the LymS, which includes symptoms of pain, swelling, fever, night sweats, itching, trouble sleeping, fatigue, weight loss, loss of appetite, trouble concentrating, and other patient concerns, also improved significantly during treatment (Figure). Additionally, FACT-G, FACT-Lym TOI, and FACT-Lym total scores were all significantly (P <.05) improved for patients receiving ruxolitinib treatment compared with BAT.
Most EORTC QLQ-C30 and FACT-Lym scores improved significantly on ruxolitinib compared with BAT. The treatment effect between the high-risk and intermediate 2-risk prognostic groups was not significantly different based on an analysis of the risk group–by–treatment interaction.
These additional analyses from the COMFORT-II study further support that ruxolitinib significantly improves overall HRQoL and MF symptoms compared with BAT.
Harrison:Novartis: Honoraria; Incyte: Honoraria; S*Bio: Honoraria; Celgene: Honoraria; Sanofi Aventis: Honoraria. Kiladjian:Novartis: Honoraria; Celgene: Honoraria. Gisslinger:Novartis: Speakers Bureau; Celgene Austria: Research Funding, Speakers Bureau; Aop-Orphan: Speakers Bureau. Knoops:Novartis: Consultancy. Waltzman:Novartis: Employment. Mendelson:Novartis: Employment, Equity Ownership. Zhou:RTI-HS: Employment; Novartis: Research Funding. Copley-Merriman:RTI-HS: Employment; Novartis: Research Funding. Hunter:Incyte Corporation: Employment, Equity Ownership. Levy:Incyte Corporation: Employment, Equity Ownership. Cervantes:Bristol-Myers-Squibb: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Passamonti:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Vannucchi:Novartis: Honoraria. Barosi:Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal