Abstract
Abstract 870
RUNX1 is generally considered a tumor suppressor in myeloid neoplasms. Blocking RUNX1 function has been implicated in development of core-binding factor (CBF) leukemia and MLL-rearranged leukemia. In addition, inactivating RUNX1 mutations have frequently been found in patients with myelodysplastic syndrome (MDS), myeloproliferative neoplasm (MPN), and cytogenetically normal acute myeloid leukemia (AML). However, no somatic RUNX1 alteration was found in CBF- and MLL-rearranged leukemias, raising the possibility that a certain level of RUNX1 activity is required for efficient propagation of these leukemia cells.
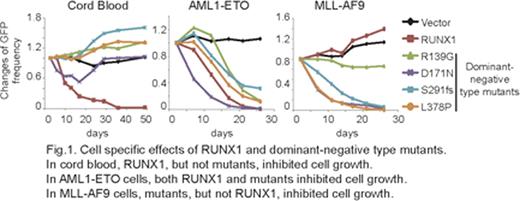
To determine the precise role of RUNX1 in specific types of myeloid neoplasms, we assessed RUNX1 functions in primary human CD34+ cord blood cells and those transduced with CBF related fusion oncoproteins [AML1-ETO (AE) or CBFB-MYH11 (CM)] or a MLL fusion oncoprotein, MLL-AF9 (MA9). RUNX1 was abundantly expressed and phosphorylated in AE-, CM-, and MA9-expressing long-term cultured cells. RUNX1 overexpression induced myeloid differentiation in normal CD34+ cells and prevented their long-term proliferation. Leukemogenic RUNX1 mutants lost the ability to induce differentiation, and a C-terminal truncated RUNX1 mutant conferred long-term (over 3 months) proliferative ability to CD34+ cells. RUNX1 overexpression also induced differentiation in CBF leukemia cells (AE- or CM-expressing cells). Interestingly, block of proper RUNX1 function, either by shRNA driven knockdown or forced expression of dominant-negative type mutants, showed growth inhibitory effects on CBF leukemia cells, suggesting that a certain level of RUNX1 activity is required for CBF leukemogenesis.
Strikingly, block of RUNX1 function, but not RUNX1 overexpression, resulted in substantial growth inhibition of MA9 cells through enhanced apoptosis and cell cycle arrest. A xenotransplantation assay further demonstrated that RUNX1 knockdown inhibited human AML development by MA9 in vivo. The growth inhibitory effect of shRNA-mediated RUNX1 knockdown on MA9 cells was rescued by reintroduction of RUNX1, and partially restored by another RUNX transcription factor RUNX2. Thus, RUNX proteins have a growth-promoting role during MA9-driven leukemogenesis. These results contrast with those obtained using a mouse transplantation model that showed loss of Runx1 accelerates the development of MLL-ENL driven leukemia. The cause of this discrepancy is unclear, but it could be explained by species differences (human vs mouse), different experimental assays (homologous transplantation vs xenotransplantation), or the compensatory mechanism of Runx1 deletion with other Runx proteins (Runx2 and Runx3) in Runx1 knockout mice. Further studies will be needed to determine the precise roles of RUNX1 in human MLL leukemias.
Finally, we assessed molecular changes in RUNX1-depleted MA9 cells and found CDKN1A upregulation and BCL2 downregulation. We also confirmed that CDKN1A depletion and BCL2 overexpression have growth-promoting effects on MA9 cells. Therefore, it appears that these molecular changes contribute to the attenuated growth of RUNX1-depleted MA9 cells. However, MA9 cells with CDKN1A depletion or BCL2 overexpression were not fully rescued from the effects of RUNX1 depletion, indicating the importance of other RUNX1 targets to support cell survival and proliferation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal