Abstract
Abstract 897
Sickle cell disease is a complex process involving biophysical and biological phenomenon such as microvascular occlusion due to rigid sickle erythrocytes, hemolysis, and aberrant cellular interactions involving endothelial cells and sickle erythrocytes and leukocytes. Indeed, a key aspect of sickle cell pathophysiology is endothelial cell dysfunction. Cardiovascular research in recent years has shown that endothelial cells biologically respond to the local mechanical environment, particularly to the changes in the applied shear stresses (Chiu and Chien, Physiological Reviews, 2011). Interestingly, no studies investigating how the biophysical alterations in sickle cell disease may directly affect endothelial function have been published. The classic view has been that vaso-occlusion is simply due to sickled erythrocytes becoming stuck in microvasculature at low oxygen tensions leading to decreased blood flow and tissue ischemia. However, the mechanical aspects of sickle cell vaso-occlusion themselves, that is, the physical phenomenon of sickling erythrocytes tightly packed in an occluded blood vessel, may directly affect endothelial biology and lead to dysfunction. We hypothesize that these pathologic forces induced by sickling erythrocytes directly lead to dysfunction of endothelial cells, which are mechanosensitive, and contribute to sickle cell pathophysiology. However, these sickling-induced forces and their effects on endothelial cells have been difficult to measure, in part due to a lack of available tools.
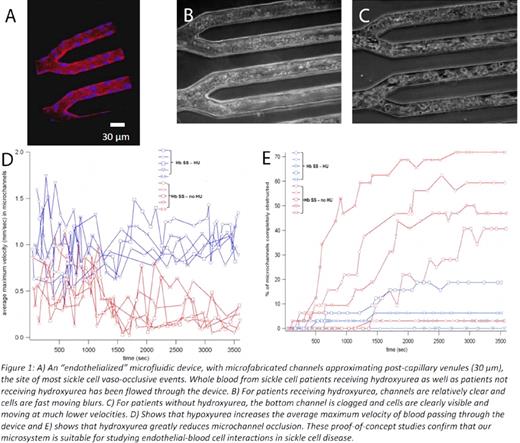
To that end, we have developed two microfluidic tools to assess the role of sickle-cell vaso-occlusion on endothelial cells. The first device is an in vitro microfluidic platform featuring microchannels the size of post-capillary venules (30 μm) with human endothelial cells cultured within and completely lining the entire inner surface of those microchannels (Figure 1A). This “microvasculature-on-a-chip” enables the visualization of blood cell-endothelial cell interactions during vaso-occlusion under a controlled hemodynamic environment and provides a platform to study the effect of vaso-occlusion on endothelial cells. To date we have characterized this “endothelialized” microfluidic device, showing that endothelial cells are confluent using anti-VE-cadherin immunostaining and adequately generate nitric oxide. Furthermore, we have flowed blood samples from patients with sickle cell disease and found that hydroxyurea treatment both reduces the number of occlusions and increases the mean velocity of the blood traveling through the device, as expected (Figure 1B–E).
To decouple whether it is a biochemical or biophysical phenomenon that causes endothelial cell dysfunction during vaso-occlusion, a second micromechanical device was created to quantitatively measure the forces generated by sickling events. The device captures whole blood and will deform outward when forces are applied by the sickle erythrocytes as shown in Figure 2. The membrane above the sickle cells has been coated with 2 μm fluorescent beads which will change focus during deflection. Deflections of one or two beads indicates that a single sickle cell is locally applying force, whereas deflections of large numbers of beads indicates that the cells are collectively applying a pressure to the membrane. The device has been fully fabricated and loaded with blood cells. An accompanying experimental setup enabling the deoxygenation of the device coupled with microscopy has also been created and preliminary tests show successful deoxygenation of sickle erythrocytes from patients with hemoglobin SS disease and the Berkeley sickle cell mouse model.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal