Abstract
Abstract 969
Selection of the appropriate therapy for patients with myelodysplastic syndromes (MDS) depends heavily on the predicted prognosis of each afflicted individual. Prognostic scoring systems help stratify patients into risk groups, but outcomes can be highly variable even within these groups. Of particular concern are patients predicted to have lower risk disease that go on to progress more rapidly than expected. Such patients may not be offered risk-appropriate therapy at a time when it might be of greatest benefit. A prognostic model that better predicts survival in patients believed to have lower risk disease has been proposed by investigators at the MD Anderson Cancer Center, but not yet validated in an independent cohort. Acquired genetic mutations can also identify patients with higher-than-predicted disease risk. We have previously demonstrated that mutations in any of five genes (TP53, EZH2, ETV6, RUNX1, and ASXL1) predict a poorer prognosis independently of the International Prognostic Scoring System (IPSS). In this study, we examined 289 MDS patients with Low or Intermediate-1 IPSS risk for mutations in 21 genes, including two genes that have recently been reported to be frequently mutated in MDS: DNMT3A and SF3B1. We validate the ability of the Lower-Risk MD Anderson Prognostic Scoring System (LR-PSS) to more finely risk-stratify patients using an independent cohort and identify gene mutations independently associated with clinical features and overall survival.
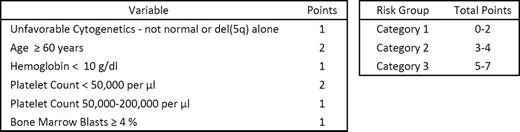
Patients were stratified into one of three risk categories using the LR-PSS shown in the Table. The 58 patients (20%) assigned to Category 1 had a median survival of 5.19 years (95% confidence interval in years [CI] 3.05–10.34), compared to 2.65 years (CI 2.18–3.30) for the 160 patients (55%) in Category 2, and 1.11 (CI 0.82–1.51) for the 71 patients (25%) in Category 3. Differences in survival were significant between all three categories (p < 0.001 for all comparisons). Point mutations were identified in 63% of samples, including 64% of those with a normal karyotype. The 10 most frequently mutated genes were TET2 (23% of cases), SF3B1 (21%), ASXL1 (15%), DNMT3A (14%), RUNX1 (9%), EZH2 (8%), JAK2 (3%), NRAS (3%), TP53 (2%), and ETV6 (2%). Mutations of SF3B1 were highly enriched in cases of refractory anemia with ring sideroblasts (RARS; 32 out of 43, 74%), associated with normal blast percentages (p = 0.04) and neutrophil counts (p = 0.002), and more likely to be present in cases with platelet counts greater than 450,000/μl (p < 0.001). We extended our analysis of SF3B1 mutations by adding a separate cohort of 98 RARS patients with Low or Intermediate-1 IPSS risk for a total of 141 cases. In this extended RARS set, SF3B1 mutations were associated with improved survival even after adjustment for IPSS risk group or LR-PSS category (hazard ratio [HR]=0.49; CI 0.29–0.81, HR=0.35; CI 0.21–0.58, respectively). SF3B1 is the first gene mutation independently associated with a favorable prognosis in non-CMML MDS. In contrast, DNMT3A mutations were not associated with differences in overall survival in the 289 patients with lower IPSS risk MDS. In a model generated from stepwise Cox regression analysis that considered LR-PSS risk categories and the mutation status of the 13 most frequently mutated genes as candidates, only EZH2 mutations emerged as a LR-PSS-independent risk factor associated with a poor prognosis (HR=2.90; CI 1.86–4.53). In a similar model using IPSS risk groups, EZH2 (HR=2.85; CI 1.78–4.57), NRAS (HR=2.78; CI 1.35–5.72), and ASXL1 (HR=1.60; CI 1.09–2.34) were significant IPSS-independent risk factors. Mutations in genes such as ASXL1, RUNX1, NRAS, and ETV6, which are associated with poor survival in unselected MDS patients, were most common in patients assigned to the LR-PSS risk Category 3, indicating that this prognostic model may capture more clinically relevant information associated with adverse gene mutations.
The Lower-Risk MD Anderson Prognostic Scoring System (LR-PSS)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal