Abstract
Abstract 972
The IPSS published in 1997, based on cytogenetics, marrow blast % and the number of cytopenias, has played a major role in prognosis assessment in MDS. A recently presented (Greenberg, International MDS Workshop, Edinburgh 2011) IPSS provisional update (IPSS-R), using the same parameters but 5 rather than 3 cytogenetic subgroups (Schanz et al, EHA 2010), a new cut off for ANC (0.8 G/L) and different weighing of parameters, appears to refine IPSS prognostic value but, like the original IPSS, was established in pts who had received no disease modifying drugs. We assessed the prognostic value of IPSS-R in 265 higher risk MDS treated with AZA, a drug with a survival impact in those pts (Lancet Oncol, 2009).
Between Sept 2004 and Jan 2009, before drug approval in EU, we enrolled 282 IPSS high and int 2 (higher) risk MDS in a compassionate patient named program of AZA and established in this cohort a prognostic scoring system (“AZA predictive score” based on Performance status (PS), cytogenetics, presence of circulating blasts, and RBC transfusion dependency) (Itzykson, Blood, 2011). We analyzed in this cohort the prognostic impact of IPSS-R in higher risk MDS treated with AZA.
Median age was 71 years. WHO diagnosis: 4% RA, RA RARS or RCMD, 20% RAEB-1, 54%RAEB-2, 22% RAEB-t (AML 20–30% blasts). Cytogenetics could be reclassified using new IPSS-R cytogenetic groups in 265 pts, in: 1% very good, 37% good, 18% int, 12% poor and 32% very poor. 66% pts had Hb<10g/dl, 74% had Platelets <100 G/L and 45% had ANC<0.8 G/l. Marrow blast % was <5%, 5–10%, 11–20%, 21–30% in 6%, 22%, 54% and 18% pts. Overall R-IPSS in the 265 patients was very good (no pt), Good (2 pts, <1%), intermediate (int 9.5%) poor (23%) and very poor (67% pts). The 2 pts of the Good group were excluded from further analysis.
56%, 55% and 38% had a response (CR, PR, or Hematological improvement- HI) to AZA in the int, poor and very poor R-IPSS groups, respectively (p=0.029). However, individual IPSS-R parameters, including IPSS-R cytogenetic classification (p=0.39), Hb level (p=0.42), platelet count (p=0.10), ANC (p=0.25) and marrow blast % (p= 0.129) stratified according to R-IPSS had no significant impact on AZA response.
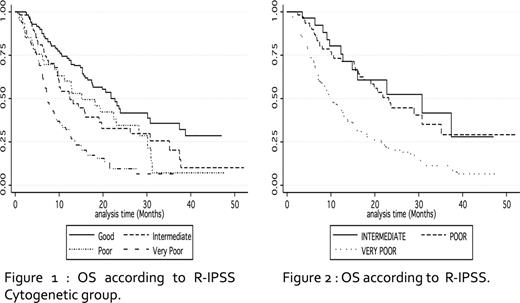
According to IPSS-R cytogenetic classification, median OS was 22.6 mo, 12.3 mo, 15.1 mo and 7.1 mo in the good, int, poor and very poor risk groups respectively (overall p <10−4, Int vs poor: p=0.85, figure 1).
According to IPSS-R, median OS was 30.7 mo, 23.1 mo and 10 mo in the int, poor and very poor R-IPSS groups respectively (Int vs poor: p=0.7; poor vs very poor: p<10−3), figure 2). Thus, there was no OS difference between patients with Int and Poor R-IPSS, who represented 32% of the patients. Among those 85 patients, however, 5%, 70% and 25% respectively could be assigned to the high, intermediate and low risk groups of our AZA predictive score (Itzykson, Blood 2011) with significant differences in OS between the 3 groups (median 6.1 mo, 21 mo and not reached, respectively, p= 0.0001). Our AZA predictive score was also valid in the Very Poor R-IPSS group, with a median survival of 5.9 mo, 12.3 mo and not reached (p<10−4) in the high, int, low risk respectively.
Using the “classical” IPSS, high and Int-2 patients treated with AZA had significantly different Response (37% vs 49%, p=0.05) and OS (median 9.4 vs 16 mo, respectively, p=0.004).
In high and int 2 risk MDS (with current IPSS) treated with AZA, the R-IPSS could predict response to AZA but found no different OS between intermediate and Poor risk patients, who represented one third of the population. Thus, refined prognostic systems established in untreated patients may have to be complemented by more specific scores when using disease-modifying drugs, like our AZA predictive score in AZA treated patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal