Abstract
Lenalidomide has demonstrated clinical activity in patients with newly diagnosed or relapsed MM, however nearly a third of relapsed patients fail to respond to it. While preclinical studies have reported variable biomarkers and pathways (Wnt-GSK3b-beta catenin; eIF4E-C/EBPb-IRF4; Cul4A-DDB1-Cereblon) as mediating the anti-MM effects of IMiDs, a comprehensive risk score for prediction of response to Lenalidomide is lacking. MiRNA are highly preserved non-coding RNAs that act post-transcriptionally to regulate gene expression by binding to the 3'UTR of mRNAs. To date studies have reported selective miRNAs expression in plasma cells at different stages of the clonal disease progression (MGUS to MM), correlated miRNA expression with distinct MM molecular subgroups and demonstrated a genome-wide elevated expression of miRNAs in high-risk MM. Herein, we have conducted a comprehensive profiling of miRNA and mRNA expression in Lenalidomide treated MM patients and established a miRNA-based risk score that is predictive of response to therapy.
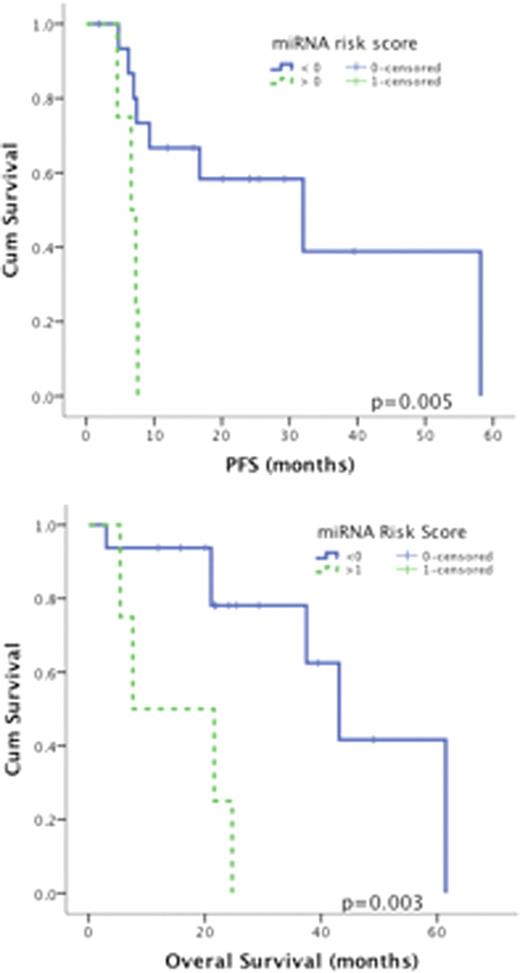
We have postulated that a miRNA signature in MM is predictive of response to Lenalidomide based therapy. To test this hypothesis, we analyzed in a testing cohort (n=20) the miRNA and mRNA signatures of Lenalidomide sensitive “S” and resistant “R” MM patients. In order to account for the role of the bone marrow environment in this disease, RNAs were extracted from 1mm punched biopsies of non-sorted and plasma cells enriched areas of the bone marrows collected immediately prior to initiating Lenalidomide. MiRNAs were hybridized to the miRNA Affymetrix gene-chip and raw miRNA expression values were log2 transformed and normalized (miRNA-QC tool, Affymetrix). Comparison of normalized miRNAs expression in “S” versus “R” patients (Anova testing) identified 29 differentially expressed miRNAs (Fold change < −2 or > 2 with a p value and FDR <0.01) between these two groups. In order to establish a risk score (RS) for prediction of response to Lenalidomide, we performed a stepwise canonical discriminant analysis with response to Lenalidomide as grouping variable and the 29 differentially expressed miRNA as independent variables. The discriminant analysis identified a RS based on the log2-scale expression of 4 miRNAs using the following equation: RS= ((1.068*hsa-miR-21) + (1.367*hsa-miR-26b) +(1.761* has-miR-3147)+(3.523*has-miR-34a) – 36.692). This univariate summary (ie, RS) of the miRNA expression profiles for each patient enabled accurate (100%) prediction of response to Lenalidomide. All patients with a RS < 0 were sensitive to Lenalidomide with a mPFS and mOS of 21.4 and 39.5 months respectively. In contrast, all patients with a RS > 0 were resistant to Lenalidomide with a mPFS and mOS of 4.1 and 24 months respectively. Validation of this RS was also performed in an independent cohort (n=20) of MM patients treated with lenalidomide. In this validation cohort the miRNA RS accurately predicted response to therapy in 90% of the cases. The mPFS and mOS were 32.0 and 43.1 months in patients with a RS < 0 (predicted as responders) as opposed to a mPFS and mOS of 6.6 and 7.6 months in patients with a RS > 0 (predicted as non-responders) (Figure below).
mRNA profiling (U133A Plus2 array chip) was also performed on the plasma cells enriched bone marrow sections with 554 genes identified as differentially expressed (Fold change < −2 or > 2 with a p value and FDR <0.005) between Lenalidomide S and R patients. Using the TargetScan miRNA target mRNA prediction tool, combinatory analysis of miRNA and mRNA expression profiles of these MM patients identified positive and negative correlations (p<0.05) between differentially expressed miRNA and mRNAs. Lastly in a multivariate Cox regression analysis that included ISS stage, FISH cytogenetics ((del17p and t(4;14)) and the 4 miRNA RS, these variables were independent predictors of survival post Lenalidomide based therapy.
We believe that this miRNA Risk Score provides a robust method of predicting sensitivity or resistance to Lenalidomide in MM patients and warrants further validation in a larger prospective study. The biological functions of these 4 miRNAs and their regulation of MM cells sensitivity to Lenalidomide is currently being investigated in vitro in a library of MM cell lines.
Neri:Celgene: Honoraria, Research Funding. Belch:Celgene: Research Funding; Onyx: Research Funding. Bahlis:Celgene: Honoraria, Speakers Bureau.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal