Abstract
Autoimmune lymphoproliferative syndrome (ALPS) represents a failure of apoptotic mechanisms to maintain lymphocyte homeostasis, permitting accumulation of lymphoid mass and persistence of autoreactive cells that often manifest in childhood with chronic nonmalignant lymphadenopathy, hepatosplenomegaly, and recurring multilineage cytopenias. Cytopenias in these patients can be the result of splenic sequestration as well as autoimmune complications manifesting as autoimmune hemolytic anemia, immune-mediated thrombocytopenia, and autoimmune neutropenia. More than 300 families with hereditary ALPS have now been described; nearly 500 patients from these families have been studied and followed worldwide over the last 20 years by our colleagues and ourselves. Some of these patients with FAS mutations affecting the intracellular portion of the FAS protein also have an increased risk of B-cell lymphoma. The best approaches to diagnosis, follow-up, and management of ALPS, its associated cytopenias, and other complications resulting from infiltrative lymphoproliferation and autoimmunity are presented. This trial was registered at www.clinicaltrial.gov as #NCT00001350.
Introduction
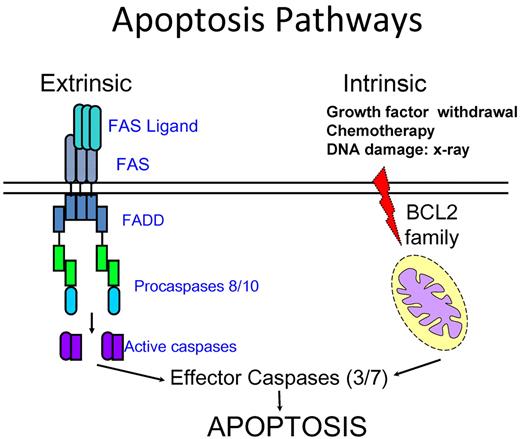
Autoimmunity results from failure of self-tolerance, which can be further divided into central and peripheral tolerance. Central tolerance is fostered by apoptosis through elimination of autoreactive lymphocytes in generative lymphoid organs (the bone marrow and thymus), whereas mechanisms of peripheral tolerance include anergy, deletion by apoptosis, and suppression by regulatory T cells to avoid autoimmunity and tissue damage.1 Apoptosis, the intrinsic program of cell death, is triggered by receptor-ligand interactions at the cell surface (the extrinsic pathway) or by the activation of mitochondrial proteins (the intrinsic pathway), leading to processing and activation of caspases and their downstream targets. Lymphocyte apoptosis mediated by the cell surface receptor FAS plays a pivotal role in lymphocyte homeostasis. FAS (also termed CD95/APO1) is a member of the tumor necrosis factor receptor superfamily of proteins that directly trigger apoptosis to maintain lymphocyte homeostasis and peripheral immune tolerance and prevent autoimmunity.2,3 It is a membrane-bound molecule that is highly expressed, not only on activated B and T lymphocytes but also present in other cells, such as hepatocytes. FAS is present on the cell surface as a preformed trimer.4 Its binding with FAS ligand leads to conformational changes on the intracellular portion of FAS protein triggering rapid recruitment of the death domain of the adaptor protein FADD (Fas-associated death domain) to the homologous death domain in the cytoplasmic tail of FAS. This is followed by the recruitment of procaspases 8 or 10 through the interaction of their death-effecter domains with the amino termini of FADD. The resulting Fas/FADD/caspase complex is termed the death-inducing signaling complex that incites a further cascade of caspase activation culminating in the death of cells (Figure 1).5,6
Schematic diagram of current understanding of extrinsic and intrinsic apoptosis pathways. The prototypical receptor of the extrinsic pathway is FAS. It recruits the adaptor FADD and the procaspases 8 and 10 on ligation. The caspases are then cleaved to further activate other downstream caspases, leading to cell death. The intrinsic pathway is controlled by proteins of the BCL2 family and triggered by stimuli, such as DNA damage and growth factor withdrawal. These stimuli ultimately lead to activation of caspase 9 and downstream effecter caspases. There is a crosstalk between the 2 pathways in some cell types.
Schematic diagram of current understanding of extrinsic and intrinsic apoptosis pathways. The prototypical receptor of the extrinsic pathway is FAS. It recruits the adaptor FADD and the procaspases 8 and 10 on ligation. The caspases are then cleaved to further activate other downstream caspases, leading to cell death. The intrinsic pathway is controlled by proteins of the BCL2 family and triggered by stimuli, such as DNA damage and growth factor withdrawal. These stimuli ultimately lead to activation of caspase 9 and downstream effecter caspases. There is a crosstalk between the 2 pathways in some cell types.
The role of FAS in maintaining lymphocyte homeostasis and peripheral immune tolerance to prevent autoimmunity was first elucidated by studies in Fas-deficient MRL/lpr−/− mice. MRL mice homozygous for Fas mutations develop hypergammaglobulinemia, glomerulonephritis, massive lymphadenopathy, and expansion of an otherwise rare and unique population of TCR-αβ+ cells that lack expression of both CD4 and CD8, and hence known as the double-negative T cells (DNT cells).7 Despite being a hallmark of the disease, the role of the DNT cells in autoimmune lymphoproliferative syndrome (ALPS) is not completely understood, partly because of the constraints of working with these cells, as they remain difficult to grow in vitro.
The understanding of the role of FAS in the immune system was further refined by recent experiments demonstrating that FAS is not key to the control of the acute immune responses in cells (ie, after exposure to viral antigens); but more importantly, it is a critical regulator of immune homeostasis during chronic infections.8-10
Taken together, these studies provided insights into the pathophysiology of a similar syndrome being observed in humans that has subsequently been called the ALPS.11-14
Clinical and laboratory features
The first clinical manifestation of ALPS is chronic lymphadenopathy and/or splenomegaly in an otherwise healthy child, often recognized by the pediatrician in a well baby clinic. Symptomatic multilineage cytopenias that are also chronic and refractory are typically most severe in early childhood, paralleling the age of expansion of the lymphocyte repertoire in children, and these tend to improve in adolescents and young adults. A large proportion of ALPS patients may present initially with episodes of fatigue, pallor, and icterus associated with hemolytic anemia, spontaneous bruises, and mucocutaneous hemorrhages because of thrombocytopenia; or bacterial infections associated with neutropenia. The potential for developing multiple autoimmune as well as infiltrative lymphoproliferative disorders involving different organ systems, such as uveitis, hepatitis, glomerulonephritis, infiltrative pulmonary lesions, and encephalitis and myelitis (manifesting as aseptic meningitis) in some patients with ALPS is being recognized on their long-term follow-up over many years (Table 1).15-19
Clinical features of ALPS15
| Feature . | % . |
|---|---|
| Lymphadenopathy | 96 |
| Splenomegaly | 95 |
| Hepatomegaly | 72 |
| Splenectomy | 49 |
| AIHA | 29 |
| ITP | 23 |
| Neutropenia | 19 |
| Glomerulonephritis | 1 |
| Liver dysfunction | 5 |
| Infiltrative lung lesions | 4 |
| Eye lesions | 0.7 |
| Feature . | % . |
|---|---|
| Lymphadenopathy | 96 |
| Splenomegaly | 95 |
| Hepatomegaly | 72 |
| Splenectomy | 49 |
| AIHA | 29 |
| ITP | 23 |
| Neutropenia | 19 |
| Glomerulonephritis | 1 |
| Liver dysfunction | 5 |
| Infiltrative lung lesions | 4 |
| Eye lesions | 0.7 |
ITP indicates immune-mediated thrombocytopenia.
The most common laboratory findings include the presence of cytopenias secondary to autoimmune destruction or splenic sequestration and polyclonal hypergammaglobulinemia. Anemia is nearly universal because of a combination of factors, including hypersplenism, autoimmunity, and iron deficiency. Eosinophilia and monocytosis are also frequent findings in patients with ALPS, although the exact pathophysiologic mechanism for these is uncertain.20 Multiple autoantibodies, frequently including those against red blood cells presenting as a positive Coomb direct antiglobulin test, have been demonstrated, even in the absence of overt autoimmune disease.21,22 One instance of acquired factor VIII inhibitor antibody has been reported in an ALPS patient.23 As discussed in more detail in “Diagnosis and classification of ALPS,” serum IL-10, soluble FAS ligand, and vitamin B12 are elevated in ALPS patients with FAS mutations and are useful biomarkers. Peripheral blood immunophenotyping reveals elevation of the characteristic DNT cells, pathognomonic of ALPS.
Genetics
ALPS is one of the first well-characterized human genetic diseases of apoptosis. Autosomal dominant transmission of heterozygous germline FAS mutations accounts for the majority of ALPS cases, whereas somatic mutations in FAS limited to subsets of lymphocytes account for the second largest group of patients in our cohort.24,25 Nonetheless, pedigrees of families carrying an ALPS-associated germline mutation reveal some family members who carry the genetic defect but have mild or absent phenotypic expression of the disease.26 This suggests that other factors, including modifier genes and haploinsufficiency, may be involved in determining phenotypic expression.27,28 Interestingly, it has recently been demonstrated that the development of acquired FAS mutations in DNT cells may be a determinant factor for the appearance of clinical symptoms in some patients with milder germline FAS mutations.27 This kind of variable penetrance and expressivity is also seen in the animal models, as mice with homozygous Fas mutations in the MRL background develop full-blown disease, whereas in other genetic backgrounds it may be clinically silent or more subdued.
Some of these patients with germline FAS mutations affecting the intracellular portion of the FAS protein also have a significantly increased risk of developing Hodgkin and non-Hodgkin lymphoma, underscoring the critical role played by cell surface receptor-mediated apoptosis in eliminating redundant proliferating lymphocytes with autoreactive and oncogenic potential.29 A handful of patients have germline deleterious mutations in genes encoding FASL or CASP10.30,31
Diagnosis and classification of ALPS
The presentation of children with generalized adenopathy, splenomegaly, and autoimmune multilineage cytopenias represents a diagnostic challenge because their clinical and laboratory features overlap with those of other childhood hematologic disorders, including lymphoma, hemophagocytic lymphohistiocytosis, hereditary spherocytosis, Evans syndrome, and Rosai-Dorfman disease.32,33 Some immunologic disorders associated with autoimmune phenomena, such as common variable immunodeficiency and Wiskott-Aldrich syndrome, must be distinguished from ALPS.34 In 1999, investigators at the National Institutes of Health suggested criteria to establish the diagnosis of ALPS.35 Since then, with ∼ 500 ALPS patients studied worldwide, significant advances in our understanding of the disease led to the revisions of the diagnostic criteria and classification scheme following the first international ALPS workshop held at National Institutes of Health in 2009.36
The diagnosis of ALPS is currently based on the presence of 2 required and 6 additional criteria. Required criteria include the presence of chronic lymphadenopathy and/or splenomegaly and elevated circulating TCRαβ+ DNT cells. Additional criteria are further divided into primary and secondary categories (Table 2). An abnormal lymphocyte apoptosis assay and the presence of pathogenic mutations in genes of the FAS pathway are the primary additional criteria. Secondary additional criteria consist of elevated circulating biomarkers, characteristic histopathology, and family history compatible with ALPS. For a definitive ALPS diagnosis, a patient has to meet both required criteria and either of the 2 primary additional criteria (Table 2). A probable ALPS diagnosis can be entertained if both the required criteria and any one of the secondary additional criteria 3, 4, 5, or 6 are present. Patients with probable ALPS should be treated and monitored in the same way as patients with a definitive diagnosis, but treating physicians are advised to pursue a genetic or apoptosis assay-based diagnostic workup whenever possible.
Revised diagnostic criteria for ALPS based on First International ALPS Workshop 200936
| Required criteria |
| 1. Chronic (> 6 months), nonmalignant, noninfectious lymphadenopathy and/or splenomegaly |
| 2. Elevated CD3+ TCRαβ+CD4−CD8− DNT cells (> 1.5% of total lymphocytes or > 2.5% of CD3+ lymphocytes) in the setting of normal or elevated lymphocyte counts |
| Additional criteria |
| Primary |
| 1. Defective lymphocyte apoptosis in 2 separate assays |
| 2. Somatic or germline pathogenic mutation in FAS, FASLG, or CASP10 |
| Secondary |
| 3. Elevated plasma sFASL levels (> 200 pg/mL), plasma IL-10 levels (> 20 pg/mL), serum or plasma vitamin B12 levels (> 1500 ng/L) or plasma IL-18 levels > 500 pg/mL |
| 4. Typical immunohistologic findings as reviewed by a hematopathologist |
| 5. Autoimmune cytopenias (hemolytic anemia, thrombocytopenia, or neutropenia) with elevated IgG levels (polyclonal hypergammaglobulinemia) |
| 6. Family history of a nonmalignant/noninfectious lymphoproliferation with or without autoimmunity |
| Definitive diagnosis: Both required criteria plus one primary accessory criterion. |
| Probable diagnosis: Both required criteria plus one secondary accessory criterion. |
| Required criteria |
| 1. Chronic (> 6 months), nonmalignant, noninfectious lymphadenopathy and/or splenomegaly |
| 2. Elevated CD3+ TCRαβ+CD4−CD8− DNT cells (> 1.5% of total lymphocytes or > 2.5% of CD3+ lymphocytes) in the setting of normal or elevated lymphocyte counts |
| Additional criteria |
| Primary |
| 1. Defective lymphocyte apoptosis in 2 separate assays |
| 2. Somatic or germline pathogenic mutation in FAS, FASLG, or CASP10 |
| Secondary |
| 3. Elevated plasma sFASL levels (> 200 pg/mL), plasma IL-10 levels (> 20 pg/mL), serum or plasma vitamin B12 levels (> 1500 ng/L) or plasma IL-18 levels > 500 pg/mL |
| 4. Typical immunohistologic findings as reviewed by a hematopathologist |
| 5. Autoimmune cytopenias (hemolytic anemia, thrombocytopenia, or neutropenia) with elevated IgG levels (polyclonal hypergammaglobulinemia) |
| 6. Family history of a nonmalignant/noninfectious lymphoproliferation with or without autoimmunity |
| Definitive diagnosis: Both required criteria plus one primary accessory criterion. |
| Probable diagnosis: Both required criteria plus one secondary accessory criterion. |
Besides the presence of chronic (> 6 months), nonmalignant, noninfectious lymphadenopathy, the presence of elevated circulating TCRαβ+ DNT cells (the second required criteria) is the hallmark of this disease. Identifying the characteristic TCRαβ+ expression on the DNT cells is critical as increased TCRγδ+ DNT cells are often a common reactive feature secondary to many disorders. For a diagnosis of ALPS, a minimum of 1.5% of total lymphocytes (or 2.5% of T lymphocytes) should be TCRαβ+ DNT cells, in the setting of normal or elevated lymphocyte counts. The presence of lymphopenia invalidates this criterion, as its impact on the relative distribution of TCRαβ+ DNT cells is currently unknown. In contrast, elevation of TCRαβ+ DNT cells > 3% of the total lymphocytes (or > 5% of T lymphocytes) is seldom seen in any conditions other than ALPS.37,38
One of the primary additional criteria is an abnormal lymphocyte apoptosis assay. However, it is no longer considered essential for the diagnosis of ALPS, as patients with both somatic FAS mutations and germline FASL mutations can present with normal in vitro FAS-induced apoptosis assays. However, the presence of a reproducible apoptotic defect in patients who fulfill other required criteria is diagnostic of ALPS.4,39 This assay is only offered by very few specialized centers, making its routine clinical use impractical. For this reason, the presence of germline or somatic deleterious mutations in FAS, FASL, or CASP10 is considered diagnostic of ALPS-FAS, ALPS-FASL, and ALPS-CASP10, respectively. Patients who fulfill the criteria for diagnosis of ALPS with indeterminate genetic cause are classified as ALPS-U, denoting an as yet undefined category.40
Gene sequencing for ALPS is currently offered by selected commercial laboratories; however, as polymorphisms in FAS are not uncommon, a diagnostic mutation should be based on prior identification of the mutation linked to a diagnosis of ALPS or a proven functional consequence of the change in association with a new mutation. Existing databases of pathogenic FAS mutations are publicly available and can be used for diagnostic help (NCBI National Institutes of Health ALPS website http://www3.niaid.nih.gov/topics/ALPS/).
Patients with ALPS-like syndromes caused by germline mutations in CASP8 and somatic mutations in NRAS and KRAS are currently classified separately as ALPS-related apoptosis disorders (Table 3).41-43 The latter group of patients with somatic NRAS and KRAS mutations present with autoimmune phenomena, massive splenomegaly, modest lymphadenopathy, and normal or only marginally elevated TCRαβ+ DNT cells.42-44 Their lymph node histopathology is also not typical of ALPS-FAS as it lacks the characteristic paracortical expansion populated with TCRαβ+ DNT cells. In addition, these patients show abnormalities of the myeloid compartment, with chronic persistent monocytosis, mimicking juvenile myelomonocytic leukemia in otherwise asymptomatic younger patients. Hence, these patients are now classified as RAS-associated autoimmune leukoproliferative disorder.36,43
ALPS classification and distribution of different categories of patients seen and evaluated at National Institutes of Health Clinical Center as part of our current cohort
| ALPS classification . | Chronic LPD/splenomegaly . | Elevated αβ DNTs . | Apoptosis defect . | % (no.) of ALPS cases (N = 257) . |
|---|---|---|---|---|
| ALPS-FAS (germline mutation) | + | + | + | 72 (185) |
| ALPS-sFAS (somatic mutation) | + | + | ± | 0.5 (14) |
| ALPS-FASLG | + | + | + | < 1 (2) |
| ALPS-CASP10 | + | + | + | < 1 (4) |
| ALPS-U | + | + | ± | 20 (52) |
| ALPS-related apoptosis disorders | ||||
| Caspase-8 deficiency state | + | ± | ± | 2 |
| RALD (somatic NRAS and KRAS mutations) | + | ± | ± | 6 |
| ALPS classification . | Chronic LPD/splenomegaly . | Elevated αβ DNTs . | Apoptosis defect . | % (no.) of ALPS cases (N = 257) . |
|---|---|---|---|---|
| ALPS-FAS (germline mutation) | + | + | + | 72 (185) |
| ALPS-sFAS (somatic mutation) | + | + | ± | 0.5 (14) |
| ALPS-FASLG | + | + | + | < 1 (2) |
| ALPS-CASP10 | + | + | + | < 1 (4) |
| ALPS-U | + | + | ± | 20 (52) |
| ALPS-related apoptosis disorders | ||||
| Caspase-8 deficiency state | + | ± | ± | 2 |
| RALD (somatic NRAS and KRAS mutations) | + | ± | ± | 6 |
RALD indicates RAS-associated autoimmune leukoproliferative disorder.
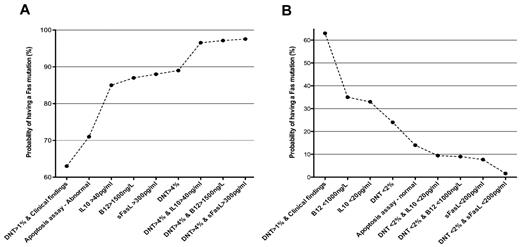
The presence of elevated TCRαβ+ DNT cells and high serum or plasma levels of IL-10, IL-18, soluble FAS ligand, or vitamin B12 can accurately predict the presence of germline or somatic FAS mutations and can be used for ALPS diagnosis.37,45 These biomarkers can predict a mutation in FAS with a post-test probability ranging from 85% to 97%, depending on the biomarker used and the number of TCRαβ+ DNT cells. The use of any of these biomarkers as diagnostic criteria greatly facilitates the diagnosis in settings that lack access to advanced genetic sequence analysis or functional testing of cell biology (Figure 2).
Signature biomarkers of ALPS-FAS. Biomarkers are very useful to predict the presence of FAS mutations in patients with features of ALPS.37 The increasing (A) or decreasing (B) post-test probabilities of having an FAS mutation in patients with different combinations of biomarkers.
Signature biomarkers of ALPS-FAS. Biomarkers are very useful to predict the presence of FAS mutations in patients with features of ALPS.37 The increasing (A) or decreasing (B) post-test probabilities of having an FAS mutation in patients with different combinations of biomarkers.
Autoimmune cytopenias and hypergammaglobulinemia in patients with lymphoproliferation associated with elevated TCRαβ+ DNT cells indicate a high likelihood of ALPS. Characteristic lymph node histopathologic findings are also helpful toward a diagnosis of ALPS-FAS.46 These include paracortical expansion because of infiltration by polyclonal TCRαβ+ DNT cells accompanied by follicular hyperplasia and polyclonal plasmacytosis. Marked TCRαβ+ DNT cell infiltration in some cases can lead to architectural effacement of lymph nodes and infiltrative changes in bone marrow and spleen, leading in some instances to an erroneous diagnosis of peripheral T-cell lymphoma. The diagnostic workup for ALPS after a lymph node biopsy should include flow cytometry, immunohistochemical evaluations, or molecular studies that rule out clonal B- and T-cell population. Finally, a positive family history for nonmalignant and noninfectious lymphadenopathy and/or splenomegaly with or without autoimmune cytopenias is helpful because many ALPS patients have family members with similar clinical histories.47
Risk of lymphoma transformation in ALPS
Physiologic apoptosis is critical in tumor surveillance as FAS, a putative tumor suppressor, is silenced in many tumors.48-50 In one report, 20% of B-cell lymphomas derived from (post) germinal center B cells carried somatic mutations in the exon 9 of the FAS gene coding for the intracellular death domain of the protein. Somatic CASP10 gene mutations were noted in 14.5% of non-Hodgkin lymphoma (NHL) in another study.51 The risk of an ALPS patient developing Hodgkin lymphoma (HL) is estimated at 50 times that of the general population, and the risk of NHL is increased 14-fold in them.29 The ALPS-associated lymphoma cohort at the National Institutes of Health Clinical Center currently consists of 20 patients (15 males and 5 females) from 15 families. Their median age at lymphoma diagnosis was 17 years (range, 5-50 years). Eleven patients had HL, and 9 patients had B cell NHL. Eighteen of 20 patients had germline heterozygous mutations of the FAS gene affecting the intracellular portion of the protein, and 2 patients have had no mutation identified. Most ALPS patients with lymphoma respond to conventional multiagent chemotherapy and radiation. Four patients (3 with HL and 1 with NHL) died because of progressive disease, and one of them developed histiocytic sarcoma after HL.52 This underscores the importance of surveillance for lymphoma in families with ALPS patients. Conversely, ALPS should also be suspected as a possible diagnosis in patients with a history of previous lymphoma presenting with nonmalignant lymphadenopathy and increased DNT cells in peripheral blood and lymph node immunohistochemistry during follow-up. Associated personal or family history of childhood onset autoimmune cytopenias, lymphadenopathy, and splenomegaly should be sought in all such patients to rule out ALPS.
Principles of management at presentation
Genetic counseling and lymphoma surveillance
Genetic counseling is an integral part of the evaluation of a family with ALPS patients in our clinic. Often, there is more than one affected person in a family. Assessment of relatives of ALPS probands with mutations in genes encoding FAS or CASP10 usually identifies a parent, sibling, or a more distant relative with identical heterozygous mutations inherited in an autosomal dominant fashion. Many such subjects share clinical features of ALPS with family members incumbent on the variable penetrance of a given disease causing gene alteration.47 Elevation in DNT cell numbers, serum vitamin B12, and IL-10 levels are typically not present in the absence of the clinical stigmata of ALPS. Patient and family members are educated in our clinic to seek timely help for any systemic symptoms, flare-ups of cytopenias, or unexpected focal fluctuations in lymph node and spleen size.
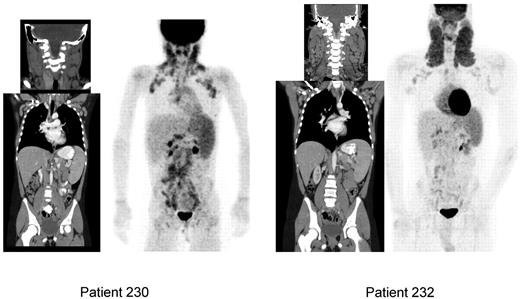
Chronic generalized adenopathy in ALPS patients can fluctuate over time in its size by up to 20% to 30% and create some concern of evolving lymphoma if one or more regional group of nodes enlarges unusually. Hence, these patients need close clinical observation and have been followed with serial CT and positron emission tomography (PET) scans every 2 to 3 years in our clinic. Some of them may have to undergo biopsy whenever there is clinical suspicion for lymphoma based on systemic symptoms and focal exacerbation of adenopathy. Noninvasive modalities of assessment are desirable in ALPS to determine whether a biopsy is warranted and which of the many enlarged nodes will probably yield informative tissue. PET using [18F] fluoro-2-deoxy-D-glucose (FDG) uptake, as a measure of cellular glucose metabolism, has become a standard in the staging and follow-up evaluation of cancers, including lymphoma. We are exploring the value of whole-body FDG-PET scan to determine whether qualitative or quantitative FDG localization can help differentiate ALPS patients with benign, albeit prominent, adenopathy from those with ALPS-associated lymphomas. Although the biodistribution of FDG in patients with ALPS is abnormal, it may still be possible to discriminate between ALPS-associated adenopathy and ALPS-associated lymphoma in a person (Figures 3 and 4) based on the clinical circumstances.
CT and FDG-PET scans featuring ALPS-FAS–associated lymphadenopathy and splenomegaly. Patient 230 is a 10-year-old girl, with asymptomatic adenopathy and splenomegaly. Patient 232 is a 22-year-old man, with asymptomatic and visible cervical and axillary lymphadenopathy and modest splenomegaly. No intervention was indicated in both patients. Note the increased uptake in the spleen as a reflection of lymphoproliferation compared with liver in both patients.
CT and FDG-PET scans featuring ALPS-FAS–associated lymphadenopathy and splenomegaly. Patient 230 is a 10-year-old girl, with asymptomatic adenopathy and splenomegaly. Patient 232 is a 22-year-old man, with asymptomatic and visible cervical and axillary lymphadenopathy and modest splenomegaly. No intervention was indicated in both patients. Note the increased uptake in the spleen as a reflection of lymphoproliferation compared with liver in both patients.
More illustrative examples of FDG-PET and CT scan appearances of some ALPS-FAS patients show splenomegaly and lymphadenopathy. Patient 004 was a 25-year-old man who presented with fever, mouth ulcers, and neutropenia; suspected lymphoma was ruled out after these scans and biopsy of cervical lymph node. The patient underwent splenectomy for persistent cytopenias. Patient 072 was a 12-year-old asymptomatic boy with cervical and axillary adenopathy. Patient 161 was a 9-year-old boy treated with MMF for chronic cytopenias for the last 8 years; 3-dimensional CT reconstruction shows enlarged spleen with volume measured at 1972 cm3. Patient 317 is a 22-year-old man with splenomegaly; CT scan shows a spleen spanning 27 cm. Bottom panel: Chest CT scan appearance in a 19-year-old man (ALPS patient 109) with otherwise asymptomatic nodular lymphocytic pulmonary infiltrates. This patient has also been on therapy for his chronic cytopenias with MMF for the last 7 years.
More illustrative examples of FDG-PET and CT scan appearances of some ALPS-FAS patients show splenomegaly and lymphadenopathy. Patient 004 was a 25-year-old man who presented with fever, mouth ulcers, and neutropenia; suspected lymphoma was ruled out after these scans and biopsy of cervical lymph node. The patient underwent splenectomy for persistent cytopenias. Patient 072 was a 12-year-old asymptomatic boy with cervical and axillary adenopathy. Patient 161 was a 9-year-old boy treated with MMF for chronic cytopenias for the last 8 years; 3-dimensional CT reconstruction shows enlarged spleen with volume measured at 1972 cm3. Patient 317 is a 22-year-old man with splenomegaly; CT scan shows a spleen spanning 27 cm. Bottom panel: Chest CT scan appearance in a 19-year-old man (ALPS patient 109) with otherwise asymptomatic nodular lymphocytic pulmonary infiltrates. This patient has also been on therapy for his chronic cytopenias with MMF for the last 7 years.
There is also a role for FDG-PET scans in postchemotherapy follow-up to rule out lymphoma relapse in the background of regenerating ALPS-related adenopathy as FDG-PET can be a valuable diagnostic tool to help choose a lymph node for biopsy based on degree of FDG avidity in ALPS patients with suspected lymphoma. ALPS-associated adenopathy recurs and must be distinguished from relapsing lymphoma in these patients.53,54
Management of lymphoproliferation and hypersplenism
Although massive, often visible, adenopathy in growing children can incite considerable anxiety and these children can be socially ostracized, treatment is not specifically indicated to shrink lymph nodes for cosmetic reasons alone. Ongoing surveillance in these patients should include careful attention to changes in lymph node size or appearance of new focal or generalized lymphadenopathy and worsening splenomegaly. The degree of generalized lymphadenopathy is documented longitudinally in a consistent fashion in our clinic. Following grading guidelines with a lymph node matrix covering all groups of nodes (eg, cervical, axillary, inguinal) are used in our clinic for longitudinal follow-up of these patients over many years: grade 1 indicates few shotty nodes; grade 2, multiple 1- to 2-cm nodes; grade 3, multiple nodes, some more than 2 cm; and grade 4, extensive visible adenopathy.55 This grading is performed using physical examination and periodic (biannual or as necessary) CT scan evaluations of neck, chest, abdomen, and pelvis (Figure 3). Clinically, splenomegaly is documented by consistently measuring its palpable extent below costal margin in the midclavicular line; nowadays, a more accurate serial volumetric assessment of splenomegaly can also be made from 3-dimensional reconstructions of CT scans (Figure 4). Neither corticosteroids nor immunosuppressive drugs, such as azathioprine, cyclosporine, or mycophenolate mofetil (MMF), reliably shrink the spleen or lymph nodes in patients with ALPS.
Spleen guards fabricated out of thermoplastic material by our Rehabilitation Medicine Department have been used with apparent success in protecting active children with large spleens from splenic rupture. This has allowed them to participate in usual school activities, including sports programs with abundant caution. Preclinical studies using MRL/lpr−/− mice have been undertaken to explore the role of different classes of drugs as lympholytic agents, including Notch signal modulators, histone deacetylase inhibitors, and arsenic trioxide.56-58 Using the immunosuppressive medication, rapamycin (sirolimus), both adenopathy and the spleen size can be reduced in MRL/lpr−/− mice and ALPS patients.59-61 However, in the latter, indication for therapy should remain relief from refractory cytopenias because of significant hypersplenism (see “Treatment of ALPS-associated refractory cytopenias”).
Splenectomy and care of its aftermath
Approximately one-half of > 250 ALPS patients being followed in our clinic have had a splenectomy to manage their chronic and refractory cytopenias. Many of them underwent the procedure before their diagnosis of ALPS could be suspected. Asplenic ALPS patients require vigilance for septicemia because of pneumococcal bacteremia, which can be fatal. Eight asplenic ALPS patients have had fatal opportunistic infections, and several have presented with pneumococcal sepsis after splenectomy. In a smaller substudy of 70 ALPS-FAS patients with adequate long-term follow-up, 34 persons (49%) from 25 families underwent splenectomy at a median age of 10 years (range, 1-53 years). Their average length of postsplenectomy follow-up was 13 years (range, 3 months to 38 years), accounting for a total follow-up of 454 person-years. The documented reasons for splenectomy included splenomegaly with cytopenias (79%), splenomegaly without cytopenias (15%), and cytopenias without splenomegaly (6%). This study also showed that ALPS-FAS patients have a high frequency of recurrence of cytopenias (56%) and sepsis (29%) after splenectomy, as 19 of 34 patients developed or relapsed with multilineage cytopenias of grade 2 or higher requiring further treatment interventions (Figure 5). Eleven patients developed sepsis 8 months to 36 years after splenectomy resulting in 2 fatalities. Many of them developed multiple septic episodes; one patient presented with 6 episodes of documented pneumococcal sepsis.62
Causes and consequences of splenectomy in a subset of 34 ALPS-FAS patients who have undergone long-term follow-up in our clinic. Note that more than half of them have relapsed with multilineage cytopenias after splenectomy requiring further therapeutic interventions, whereas one-third of them have had septic episodes.
Causes and consequences of splenectomy in a subset of 34 ALPS-FAS patients who have undergone long-term follow-up in our clinic. Note that more than half of them have relapsed with multilineage cytopenias after splenectomy requiring further therapeutic interventions, whereas one-third of them have had septic episodes.
ALPS patients may be more vulnerable to sepsis as they lack circulating CD27+ memory B lymphocyte populations.63 It may account for their inability to mount or sustain protective levels of antibodies directed against pneumococcal polysaccharide antigens after vaccination. Based on our experience, all asplenic ALPS patients should preferably remain on long-term antibiotic prophylaxis against pneumococal sepsis using penicillin V or fluoroquinolones, such as levofloxacin. In addition to advising our asplenic patients to wear Medic alert bracelets, we also educate them and their parents and guardians about the importance of seeking medical care promptly for a significant febrile illness requiring intravenous antibiotics until bacterial sepsis is ruled out. Our recommendations for asplenic ALPS patients include life-long daily antibiotic prophylaxis as well as periodic surveillance and reimmunization against pneumococci using a combination of both 13-valent conjugate (Prevnar-13) and 23-valent polysaccharide (Pneumovax) vaccines every 4 to 5 years.64,65
Recently, there has been an increased awareness of some associated morbidity and mortality, including overwhelming postsplenectomy infection, on long-term follow-up of patients after splenectomy for other indications as well.66-68 Thus, splenectomy should be avoided unless it is the only remaining measure to control chronic, refractory, life-threatening cytopenias in ALPS patients. Even under those circumstances, feasibility and efficacy of a partial splenectomy or splenic embolization should be explored.
Treatment of ALPS-associated refractory cytopenias
The initial management of patients with ALPS-related autoimmune cytopenias (autoimmune hemolytic anemia [AIHA], immune-mediated thrombocytopenia, autoimmune neutropenia) is similar to sporadic immune cytopenias in other patient populations.69 Most recently, updated immune thrombocytopenia treatment guidelines and recommendations from the American Society of Hematology (2011) can be broadly applicable to ALPS patients with chronic and persistent thrombocytopenia.70 Although long-term and careful follow-up of patients with chronic multilineage cytopenias after any treatment is necessary to determine relative risks and benefits, in our practice the following caveats do apply specifically to ALPS patients (Figure 6):
Immune suppression with corticosteroids. We use high-dose pulse therapy with intravenous methylprednisolone (5-10 mg/kg) followed by low-dose oral prednisone (1-2 mg/kg) maintenance therapy that can often be successfully tapered over several weeks (8-12 weeks) as ALPS patients often have chronic and refractory disease. Intravenous methylprednisolone at doses as high as 30 mg/kg per day for 1 to 3 days may have to be used in some patients with profoundly refractory cytopenias (eg, hemoglobin < 5 g requiring intensive care for hypoxia). However, one has to be aware of the usual steroid-related short- and long-term morbidities as Cushingoid body habitus, hypertension, cataracts, hyperglycemia, osteopenia, and avascular necrosis of the hip have been noted in some of our patients. This has prompted us to look for steroid-sparing measures (Figure 6).
Intravenous immunoglobulin G (1-2 gm/kg) given concomitantly with pulse dose methylprednisolone may benefit some patients with severe AIHA by abrogating antibody-mediated red cell destruction and allowing packed red blood cell transfusion support for severe anemia. We avoid using WinRho for isolated thrombocytopenia in ALPS patients, as many of them are DAT-positive and likely to develop hemolysis.
ALPS patients with isolated chronic neutropenia and associated infections can also benefit from twice or thrice weekly, low-dose (1-2 μg/kg) G-CSF given subcutaneously.
Standard-dose rituximab (375 mg/m2 per week 4 times) for treatment of refractory, chronic cytopenias in children has been used by others as well as in 12 ALPS patients in our cohort.71,72 In 7 of 9 patients with ALPS and thrombocytopenia, rituximab therapy led to median response duration of 21 months (range, 14-36 months). In contrast, none of the 3 children treated with rituximab for AIHA responded. Noted toxicities included profound and prolonged hypogammaglobulinemia in 3 patients requiring replacement intravenous immunoglobulin G, total absence of antibody response to polysaccharide vaccines lasting up to 4 years after rituximab infusions in one patient, and prolonged neutropenia in one other patient. These toxicities constitute an additional infection risk burden, especially in asplenic persons, and may warrant avoidance of rituximab in ALPS patients until other immunosuppressive medication options are exhausted.
Use of MMF in 13 children with ALPS, given twice daily orally at a dose of 600 mg/m2 per dose for chronic cytopenias, was initially described by us in 2005.73 This preliminary experience suggested that MMF has spared chronic steroid usage in patients with ALPS-associated cytopenias. ALPS patients, especially those with massive splenomegaly and hypersplenism, can often be refractory to standard-dose short-term corticosteroid regimens, intravenous immunoglobulin G, and packed red blood cell transfusions and may require other measures. We have used MMF in 61 ALPS patients over the past 11 years as ongoing long-term steroid-sparing immune suppression for chronic and refractory cytopenias as well as other autoimmune manifestations, such as uveitis, glomerulonephritis, and pulmonary lesions (Figure 4). Their median age is 10 years (range, 6 months to 43 years) with a median follow-up of 3 years (range, 3 months to 11 years). Fifty-six patients responded to MMF as defined by maintenance of adequate blood counts and reduction in its dosage or cessation of other immunosuppressive agents; however, 5 of them required other therapies later on as their cytopenias became more refractory, and one of them died because of relapse with a refractory, overwhelming AIHA after a period of response to MMF for 5 years. Although 16 patients had undergone splenectomy before initiating MMF, in some patients, MMF has allowed splenectomy to be avoided or postponed until the very young children had aged and would better tolerate surgical asplenia. However, it is critical to use MMF only in the context of a steroid-sparing measure and not as a first-line upfront therapy to treat significant cytopenias.
Management suggestions for ALPS-associated chronic refractory cytopenias. This schematic diagram is included only as a suggested guideline for managing children with ALPS-associated autoimmune multilineage cytopenias. Use of G-CSF may be warranted for severe neutropenia associated with systemic infections. Similarly, use of other chemotherapeutic and immunosuppressive agents besides MMF and sirolimus (eg, hydroxychloroquine, methotrexate, mercaptopurine, vincristine, azathioprine, and cyclosporine) can also be considered as a steroid-sparing measure or used while avoiding or postponing surgical splenectomy at the discretion of the treating clinicians based on the circumstances of a specific patient.
Management suggestions for ALPS-associated chronic refractory cytopenias. This schematic diagram is included only as a suggested guideline for managing children with ALPS-associated autoimmune multilineage cytopenias. Use of G-CSF may be warranted for severe neutropenia associated with systemic infections. Similarly, use of other chemotherapeutic and immunosuppressive agents besides MMF and sirolimus (eg, hydroxychloroquine, methotrexate, mercaptopurine, vincristine, azathioprine, and cyclosporine) can also be considered as a steroid-sparing measure or used while avoiding or postponing surgical splenectomy at the discretion of the treating clinicians based on the circumstances of a specific patient.
We usually suggest addition of MMF to the therapy regimen and when the patients are being treated with corticosteroids for a flare-up of their cytopenia (as reflected by a complete blood count with hemoglobin < 8 g, absolute neutrophil count < 500 cells, or platelets < 50 000) while they are being slowly tapered off their corticosteroids over 8- to 12-week period. MMF should be given concomitantly for at least 2 weeks to reach effective therapeutic drug levels in plasma while the patient is on a tapering schedule of corticosteroids (Figure 6). No evidence of increased infection risk or other significant toxicities has been noted in any of our ALPS patients on chronic MMF therapy over the last 11 years.
6. Rapamycin (sirolimus), an mTOR inhibitor, has recently been used successfully by Teachey et al as well as others in ALPS patients.61 Most ALPS patients on rapamycin show a good response, and many achieve a normal blood count for the first time in their life since infancy. A second advantage is that rapamycin reduces lymphoproliferation, as the enlarged lymph nodes and spleen often shrink significantly with reduction of the signature DNT cell numbers. However, these patients, just like the patients on MMF, need to be maintained on sirolimus for the long-term and monitored for its toxicities diligently. Many ALPS patients requiring sirolimus present with significant lymphoproliferative burden evidenced by massive splenomegaly and adenopathy. Their cytopenias should be refractory to upfront corticosteroid therapy and/or steroid-sparing measures, including MMF. After the initial loading dose of 3 mg/m2, patients should take sirolimus orally once daily at 2.5 mg/m2 per day (up to a maximum daily dose of 4 mg), to achieve a target 24-hour trough drug level of 5 to 15 ng/mL. Levels of sirolimus should be drawn at least twice per week until steady state is reached, then weekly or monthly thereafter. Children younger than 15 years may metabolize sirolimus more briskly than adults and may need twice-daily dosing to achieve targeted trough levels. However, ensuring adequate kidney and liver function while monitoring for toxic side effects of sirolimus, including T-cell immunosuppression, hypercholesterolemia, and stomatitis, is imperative through interval clinical evaluations, measurement of blood drug levels, and dose adjustment.
Before commencing medications, such as MMF or sirolimus, their risk-benefit ratio should be taken into consideration for an individual ALPS patient, as they often may have to be exposed to long-term ongoing immunosuppression to remain free from their refractory cytopenias. There have been reports of successful clinical use of other agents, including hydroxychloroquine, dapsone, azathioprine, and 6-mercaptopurine, for the relief of chronic cytopenias in children and adults, and they should also be considered as a viable option in some patients with ALPS.74,75
Role of HSCT in ALPS
Both short and long prognosis of most ALPS patients appears to be good. In our entire cohort consisting of 257 persons, only13 ALPS patients have died: 8 because of postsplenectomy sepsis, 1 because of overwhelming hemolytic anemia and toxicities of its treatment, and 4 because of progressive malignancy. The chronic cytopenias seen in many improve with age, and many of them continue responding to conventional short-term immunosuppressive treatment. Thus, there is no need to entertain allogeneic hematopoietic stem cell transplantation (HSCT) in the vast majority of patients with ALPS. However, HSCT has been reportedly successful in selected ALPS patients with lymphoma, polyarteritis nodosa, or very severe phenotype because of homozygous FAS mutation and refractory cytopenias.76-78 A few other ALPS patients have been transplanted with variable results, with most failures being the result of progression of their malignancy, ineffective engraftment resulting from nonmyeloablative conditioning, and opportunistic infections. Of course, a sibling who carries the same apoptosis pathway mutation as the proband is not an appropriate marrow donor, even if asymptomatic. In addition, mortality resulting from matched unrelated donor-derived HSCT is too high (∼ 35%) to justify this procedure for the majority of patients with a chronic disease, such as ALPS in which a near-normal life span can otherwise be expected. Although most ALPS patients get better as they grow older, some of them do require chronic long-term immunosuppression, most often for multilineage cytopenias and other end-organ damage because of lymphoproliferation or its therapy. If a patient's ALPS-associated lymphoproliferative disorder is likely to require life-long potent immunosuppression, that in itself is likely to lead to more morbidity to end organs, such as liver, kidney, and lungs. HSCT may have a role in selected ALPS patients with a more severe phenotype as they may benefit from a life-long respite from ALPS, its complications, and their therapies.47,79
Implications of ALPS and future directions
The major determinants of morbidity and mortality in ALPS depend on the severity of the autoimmune disease, hypersplenism and asplenia-related sepsis, and the development of lymphoma (Table 4). Patients with mutations abrogating function of the intracellular domain of the FAS protein are at risk of developing lymphomas, and they need diligent life-long follow-up. It is necessary to understand the natural history of this otherwise rare disorder to prognosticate which of these patients might benefit from early interventions, such as use of MMF, sirolimus, or stem cell transplantation while avoiding a splenectomy and make risk-adaptive recommendations. This decision has to be based on their underlying disease phenotype and assessment of the risk-benefit ratio of any proposed treatment regimen. The role of thrombopoietin mimetic agents and histone deacetylase inhibitors in managing ALPS-associated recalcitrant cytopenias and hypersplenism needs further exploration. Novel and nontoxic lympholytic therapies are necessary to control the lymphoproliferative process in children with ALPS who have chronic lymphadenopathy and splenomegaly. They also require long-term monitoring and collaborative follow-up by multiple subspecialists familiar with ALPS.
Salient aspects of management and prognosis of ALPS
| Diagnosis and genetic counseling at presentation |
| Significant number of ALPS patients do not need any intervention for asymptomatic lymphadenopathy and splenomegaly that often seems to get better with age. |
| Use of spleen guards made of thermoplastic material for protecting enlarged spleens from trauma |
| Avoid splenectomy |
| For unavoidable surgical asplenia: use 13 valent conjugate and 23 valent polysaccharide vaccines against pneumococcal sepsis |
| Med alert bracelet, fever alert, and long-term antibiotic prophylaxis |
| Autoimmune cytopenias: short-term steroids and IVIG |
| Steroid-sparing measures: mycophenolate mofetil and sirolimus |
| Vigilance for lymphoma: role of periodic CT and FDG-PET scans |
| Deaths (13 of 257 patients) in our cohort: death resulting from sepsis with asplenia (9), malignancies (4) |
| Diagnosis and genetic counseling at presentation |
| Significant number of ALPS patients do not need any intervention for asymptomatic lymphadenopathy and splenomegaly that often seems to get better with age. |
| Use of spleen guards made of thermoplastic material for protecting enlarged spleens from trauma |
| Avoid splenectomy |
| For unavoidable surgical asplenia: use 13 valent conjugate and 23 valent polysaccharide vaccines against pneumococcal sepsis |
| Med alert bracelet, fever alert, and long-term antibiotic prophylaxis |
| Autoimmune cytopenias: short-term steroids and IVIG |
| Steroid-sparing measures: mycophenolate mofetil and sirolimus |
| Vigilance for lymphoma: role of periodic CT and FDG-PET scans |
| Deaths (13 of 257 patients) in our cohort: death resulting from sepsis with asplenia (9), malignancies (4) |
Acknowledgments
The authors thank all patients and referring physicians for participating in the ALPS Natural History Study at National Institutes of Health Clinical Center as well as Les Folio, Millie Whatley, and Clara Chen for help with choosing the CT and PET scan images, and Susan Price, Joie Davis, and Katie Perkins for assistance with providing clinical care to the patients.
This work was supported by the Intramural Research Program (Division of Intramural Research) of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
This study has undergone annual review by the National Institute of Allergy and Infectious Diseases Institutional Review Board. All participants whose clinical data are included in this manuscript were enrolled on the study after undergoing the informed consent process.
National Institutes of Health
Authorship
Contribution: V.K.R. participated in clinical care and wrote the manuscript; and J.B.O. provided laboratory diagnostic workup and reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: V. Koneti Rao, ALPS Unit, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Dr, Rm 11N 230, Bethesda, MD 20892; e-mail: korao@niaid.nih.gov.