Abstract
We assessed efficacy, safety, and reversal of renal impairment (RI) in untreated patients with multiple myeloma given bortezomib-melphalan-prednisone-thalidomide followed by bortezomib-thalidomide (VMPT-VT) maintenance or bortezomib-melphalan-prednisone (VMP). Exclusion criteria included serum creatinine ≥ 2.5 mg/dL. In the VMPT-VT/VMP arms, severe RI (estimated glomerular filtration rate [eGFR] ≤ 30 mL/min), moderate RI (eGFR 31-50 mL/min), and normal renal function (eGFR > 50 mL/min), were 6%/7.9%, 24.1%/24.9%, and 69.8%/67.2%, respectively. Statistically significant improvements in overall response rates and progression-free survival were observed in VMPT-VT versus VMP arms across renal cohorts, except in severe RI patients. In the VMPT group, severe RI reduced overall survival (OS). RI was reversed in 16/63 (25.4%) patients receiving VMPT-VT versus 31/77 (40.3%) receiving VMP. Multivariate analysis showed male sex (P = .022) and moderate RI (P = .003) significantly predicted RI recovery. VMP patients achieving renal response showed longer OS. In both arms, greater rates of severe hematologic adverse events were associated with RI (eGFR < 50 mL/min), however, therapy discontinuation rates were unaffected. VMPT-VT was superior to VMP for cases with normal renal function and moderate RI, whereas VMPT-VT failed to outperform VMP in patients with severe RI, although the relatively low number of cases analyzed preclude drawing definitive conclusions. VMPT-VT had no advantage in terms of RI reversal over VMP. This study is registered at http://www.clinicaltrials.gov as NCT01063179.
Introduction
Renal impairment (RI) is a common feature of multiple myeloma (MM).1,2 At diagnosis 30%-40% of patients with MM have serum creatinine (sCr) levels greater than the upper normal limit but not exceeding 4 mg/dL in the majority of patients.3-5 Cast nephropathy is the main cause of RI in MM (∼ 90% of cases) and reflects advanced disease and high tumor burden status.6-10
RI was associated with poor prognosis in patients with MM in the era of conventional chemotherapy, and the authors of several studies demonstrated a median survival shorter than 2 years for these patients.11-13 Early, effective treatment can lead to the reversal of RI.1,3,14 Moreover, patients who achieve a reversal of RI have a prolonged survival compared with those with irreversible impairment.3,14
In recent years, several data support the safety and efficacy of bortezomib-based therapies in patients with myeloma with RI of any grade, with associated improvements in renal function.15-22
Limited data are available regarding the efficacy of thalidomide-based regimens in patients with MM and RI.23,24 To date, given the absence of randomized results, any additional benefit of thalidomide for patients with myeloma with RI has not been demonstrated, and thus, its use is recommended with caution and appropriate dose reduction.
Our group has recently published results of a phase 3 study examining the efficacy of the 4-drug combination of bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide (VMPT-VT) compared with bortezomib-melphalan-prednisone (VMP) treatment alone in untreated MM patients who were ineligible for autologous stem cell transplantation.25 In this trial, patients with sCr levels ≥ 2.5 mg/dL were excluded. Herein, we report on a cohort analysis in which we assessed the efficacy and safety and reversal of RI for VMPT-VT versus VMP in newly diagnosed patients with MM and RI.
Methods
Patients and study design
This study is registered at http://www.clinicaltrials.gov as #NCT01063179. Full details of the phase 3 randomized study have been published previously.25 Patients with newly diagnosed MM who were ineligible for autologous stem cell transplantation participated in the trial. Patients having sCr level ≥ 2.5 mg/dL were excluded. Experimental therapy consisted of induction with 9 cycles (6 weeks each) of melphalan 9 mg/m2 on days 1-4; prednisone 60 mg/m2 on days 1-4; bortezomib 1.3 mg/m2 on days 1, 4, 8, 11, 22, 25, 29, and 32 during cycles 1-4 and on days 1, 8, 22, and 29 during cycles 5-9; and thalidomide 50 mg/d continuously. Patients received maintenance therapy with bortezomib 1.3 mg/m2 every 14 days and thalidomide 50 mg/d for 2 years or until progression or relapse. Standard VMP therapy consisted of induction therapy with 9 cycles of VMP (6 weeks each), at the doses described previously, without maintenance.
After the inclusion of the first 139 patients, the protocol was amended to reduce the incidence of peripheral neuropathy. Both induction schedules were changed to 9 cycles (5 weeks each) and the bortezomib dose was modified to 1.3 mg/m2 on days 1, 8, 15, and 22 during cycles 1-9. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of progression, relapse, death for any cause or the date the patient was last known to be in remission. Duration of response (DOR) was calculated from the time of the attainment of response until the date of progression. Overall survival (OS) was calculated from the time of diagnosis until the date of death for any cause or the last follow-up. PFS and OS were analyzed for all patients, whereas response rates were analyzed in those patients receiving at least one cycle of study drugs. The treatment response was defined by use of the International Uniform Response Criteria.26 All adverse events were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (Version 3.0).27
Analysis of the renal cohort
In this analysis only those patients having complete monthly data for the calculation of calculated creatinine clearance (CrCl) during induction were included. Patients were subdivided by baseline estimated glomerular filtration rate (eGFR), according to CrCl28 and revised29 stratification by the National Kidney Foundation Practice Guidelines for Chronic Kidney Disease.30 Normal renal function was defined as eGFR > 50 mL/min and RI (eGFR ≤ 50 mL/min) was subdivided into moderate (31-50 mL/min) or severe (≤ 30 mL/min). Reversibility of RI was defined as improvement of eGFR from < 50 mL/min at baseline to > 60 mL/min after induction therapy. Renal response was evaluated according to the definitions of Ludwig et al31 (complete response: baseline eGFR < 50 mL/min improving to ≥ 60; partial response: baseline eGFR < 15 improving to 30 to < 60; minimal response: baseline eGFR < 15 improving to 15 to < 30 or baseline eGFR 15 to < 30 improving to 30 to < 60).31
Statistical analysis
All statistical calculations were performed by use of the statistical package SPSS for Windows (v13.0; 2004 SPSS Working). Overall response rates (ORR), CR rates, and the incidence of adverse events (AEs) was compared between arms by renal cohort with the Mantel-Haenszel estimate of common odds ratio (OR) for stratified tables, with P values determined with the Mantel-Haenszel χ2 test. For categorical variables, statistical comparisons were performed by the use of 2-way tables for the Fisher exact test and multiway tables for the Pearson χ2 test. Survival functions were estimated with the Kaplan-Meier method, and curves for categorical variables were plotted for PFS, DOR, time to RI reversal, and OS. The P values for testing the differences between subgroups and levels for each variable were calculated by log-rank test. Factors associated with RI reversal were assessed by logistic univariate and multivariate analyses. Hazard ratios (HRs) for comparisons between arms by cohort were determined on the basis of a stratified Cox regression analyses. A P value of < .05 was considered significant for all statistical calculations.
Results
Patient characteristics
Of the 511 patients originally allocated to the 2 arms,25 complete data for the calculation of monthly CrCl during induction therapy were available for 473 patients; 232 cases were randomly assigned to VMPT-VT and 241 to VMP. Approximately 30% of patients on each arm presented with RI, most with moderate RI. In particular, 14 (6%), 56 (24.1%), and 162 (69.8%) patients receiving VMPT-VT and 19 (7.9%), 60 (24.9%), and 162 (67.2%) receiving VMP had a eGFR ≤ 30, 31-50, and > 50 mL/min, respectively (P = .69); 0 and 2 patients receiving VMPT-VT and VMP, respectively, had eGFR ≤ 20 mL/min. Baseline demographic and disease characteristics, as well as the percentage of patients treated with bortezomib once or twice weekly, were balanced between arms and respective renal cohorts (Table 1). However, an expected greater proportion of patients with β2-microglobulin > 5.5 mg/L and International Staging System stage III disease were included in the more severe RI group. The subgroup of VMPT-VT patients with severe RI showed a greater, although not statistically significant, rate of high-risk cytogenetics compared with VMP patients with severe RI (VMPT-VT vs VMP: 5/12 [41.7%] cases vs 4/14 [28.5%]; P = .4).
Main clinical and laboratory characteristics of patients distributed by VMPT-VT and VMP arms and by renal cohorts
| Characteristics . | VMPT-VT arm, estimated glomerular filtration rate . | VMP arm, estimated glomerular filtration rate . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 232) . | ≤ 30 (n = 14) . | 31-50 (n = 56) . | ≤ 50 (n = 70) . | > 50 (n = 162) . | Total (n = 241) . | ≤ 30 (n = 19) . | 31-50 (n = 60) . | ≤ 50 (n = 79) . | > 50 (n = 162) . | |
| Median age, y | 71 | 74.5 | 73.5 | 74 | 70 | 71 | 72 | 74 | 72 | 71 |
| Male, % | 51.3 | 42.9 | 39.3 | 40 | 56.2 | 46.9 | 31.6 | 46.7 | 43 | 48.8 |
| KPS ≤ 70%, % | 30.6 | 35.7 | 33.9 | 34.3 | 29 | 26.6 | 31.6 | 31.7 | 31.6 | 24.1 |
| ISS stage III, % | 23.8 | 90 | 34.7 | 44.1 | 14.6 | 29.1 | 73.3 | 48.9 | 55 | 17.1 |
| Median β2M, mg/L | 3.7 | 10.3 | 4.6 | 5.1 | 3.3 | 4 | 7.2 | 5.4 | 5.97 | 3.5 |
| Median albumin, g/dL | 3.75 | 3.5 | 3.6 | 3.6 | 3.87 | 3.8 | 4 | 3.7 | 3.8 | 3.75 |
| Chromosome abnormalities* | ||||||||||
| del13, % | 53.6 | 83.3 | 53.1 | 59 | 50.8 | 46.6 | 42.9 | 46.3 | 45.5 | 47.1 |
| t(4;14), % | 16.8 | 25 | 22.4 | 23 | 13.6 | 12.6 | 7.1 | 9.8 | 9.1 | 14.3 |
| t(11;14), % | 16.2 | 16.7 | 10.2 | 11.5 | 18.6 | 11.5 | 7.1 | 9.8 | 9.1 | 12.6 |
| t(14;16), % | 5 | 8.3 | 8.2 | 8.2 | 3.4 | 3.4 | 0 | 0 | 0 | 5 |
| del17, % | 17.3 | 16.7 | 8.2 | 9.8 | 21.2 | 11.5 | 21.4 | 7.3 | 10.9 | 11.8 |
| Bortezomib schedule | ||||||||||
| Once weekly, % | 74.6 | 85.7 | 71.4 | 74.3 | 74.7 | 76.3 | 89.5 | 71.7 | 75.9 | 76.5 |
| Twice weekly, % | 25.4 | 14.3 | 28.6 | 25.7 | 25.3 | 23.7 | 10.5 | 28.3 | 24.1 | 23.5 |
| Characteristics . | VMPT-VT arm, estimated glomerular filtration rate . | VMP arm, estimated glomerular filtration rate . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 232) . | ≤ 30 (n = 14) . | 31-50 (n = 56) . | ≤ 50 (n = 70) . | > 50 (n = 162) . | Total (n = 241) . | ≤ 30 (n = 19) . | 31-50 (n = 60) . | ≤ 50 (n = 79) . | > 50 (n = 162) . | |
| Median age, y | 71 | 74.5 | 73.5 | 74 | 70 | 71 | 72 | 74 | 72 | 71 |
| Male, % | 51.3 | 42.9 | 39.3 | 40 | 56.2 | 46.9 | 31.6 | 46.7 | 43 | 48.8 |
| KPS ≤ 70%, % | 30.6 | 35.7 | 33.9 | 34.3 | 29 | 26.6 | 31.6 | 31.7 | 31.6 | 24.1 |
| ISS stage III, % | 23.8 | 90 | 34.7 | 44.1 | 14.6 | 29.1 | 73.3 | 48.9 | 55 | 17.1 |
| Median β2M, mg/L | 3.7 | 10.3 | 4.6 | 5.1 | 3.3 | 4 | 7.2 | 5.4 | 5.97 | 3.5 |
| Median albumin, g/dL | 3.75 | 3.5 | 3.6 | 3.6 | 3.87 | 3.8 | 4 | 3.7 | 3.8 | 3.75 |
| Chromosome abnormalities* | ||||||||||
| del13, % | 53.6 | 83.3 | 53.1 | 59 | 50.8 | 46.6 | 42.9 | 46.3 | 45.5 | 47.1 |
| t(4;14), % | 16.8 | 25 | 22.4 | 23 | 13.6 | 12.6 | 7.1 | 9.8 | 9.1 | 14.3 |
| t(11;14), % | 16.2 | 16.7 | 10.2 | 11.5 | 18.6 | 11.5 | 7.1 | 9.8 | 9.1 | 12.6 |
| t(14;16), % | 5 | 8.3 | 8.2 | 8.2 | 3.4 | 3.4 | 0 | 0 | 0 | 5 |
| del17, % | 17.3 | 16.7 | 8.2 | 9.8 | 21.2 | 11.5 | 21.4 | 7.3 | 10.9 | 11.8 |
| Bortezomib schedule | ||||||||||
| Once weekly, % | 74.6 | 85.7 | 71.4 | 74.3 | 74.7 | 76.3 | 89.5 | 71.7 | 75.9 | 76.5 |
| Twice weekly, % | 25.4 | 14.3 | 28.6 | 25.7 | 25.3 | 23.7 | 10.5 | 28.3 | 24.1 | 23.5 |
β2M indicates β2microglobulin; del, deletion; ISS, International Staging System; KPS, Karnofsky performance status; t, translocation; VMP, bortezomib-melphalan-prednisone; and VMPT-VT, bortezomib-melphalan-prednisone-thalidomide followed by bortezomib-thalidomide maintenance.
Data available in 179 of 232 cases of the VMPT-VT arm and in 174 of 241 cases of the VMP arm.
Efficacy
Overall, 221 and 235 patients randomly assigned to VMPT-VT and VMP, respectively, received at least one cycle of study drugs and were evaluable for response. ORR and CR rates were greater in the VMPT-VT versus VMP group (ORR, OR = 2.8, P = .001; CR rate, OR = 1.9, P = .002; Table 2). A significant advantage in favor of VMPT-VT was observed in patients with normal renal function (ORR: OR = 2.3, P = .033; CR rates: OR = 1.7, P = .023) and in those with eGFR ≤ 50 mL/min (ORR: OR = 4.2, P = .015; CR rates: OR = 2.3, P = .025). Although the VMPT-VT arm still maintained a significantly greater ORR and a trend toward better CR in the subgroup of patients with moderate RI (ORR: OR = 5.8, P = .026; CR rates: OR = 2.1, P = .07), in the smaller cohort with severe RI numerical differences were not statistically significant (ORR: OR = 2.1, P = .3; CR rates, OR = 3.0, P = .2). Within the VMPT-VT and VMP arms, ORR and CR rates appeared similar between renal cohorts. Median time to first response appeared similarly rapid in both arms and was not influenced by renal function (Table 2).
Response rate, PFS, and OS in the VMPT-VT and VMP arms and by renal cohorts
| Measure . | VMPT-VT arm, estimated glomerular filtration rate . | VMP arm, estimated glomerular filtration rate . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 232) . | ≤ 30 (n = 14) . | 31-50 (n = 56) . | ≤ 50 (n = 70) . | > 50 (n = 162) . | Total (n = 241) . | ≤ 30 (n = 19) . | 31-50 (n = 60) . | ≤ 50 (n = 79) . | > 50 (n = 162) . | |
| Response-evaluable, n | 221 | 11 | 52 | 63 | 158 | 235 | 19 | 58 | 77 | 158 |
| Response rate, % | 93.2 | 81.8a | 96.2b | 93.7c | 93d | 83 | 68.4 | 81 | 77.9 | 85.4e |
| CR rate, % | 39.4 | 36.4f | 42.3g | 41.3h | 38.6i | 25.5 | 15.8 | 25.9 | 23.4 | 26.6j |
| Median time to first response, mo | 1.4 | 1.2k | 1.4l | 1.4m | 1.4n | 1.4 | 1.4 | 1.4 | 1.4 | 1.4o |
| Median duration of response, mo | NR | 19.8p | NRq | NRr | NRs | 27.9 | 20 | 22 | 21.8 | 28.6t |
| Median PFS, mo | NR | 20.9 | NR | NR | NR | 24.6 | 22.5 | 24.2 | 24.2 | 25.1 |
| 1-year PFS, % | 93 | 80 | 96 | 96 | 92 | 87 | 83 | 89 | 87 | 87 |
| 2-year PFS, % | 69 | 40 | 73 | 69 | 69 | 55 | 46 | 57 | 54 | 56 |
| Median OS, mo | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 1-year OS, % | 92.3 | 75.2 | 94.2 | 90.7 | 93 | 93.8 | 88.9 | 93.1 | 92.1 | 95.3 |
| 2-year OS, % | 88 | 60.2 | 89.6 | 84.2 | 89.6 | 89.3 | 83.3 | 88.7 | 87.3 | 90.3 |
| Measure . | VMPT-VT arm, estimated glomerular filtration rate . | VMP arm, estimated glomerular filtration rate . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 232) . | ≤ 30 (n = 14) . | 31-50 (n = 56) . | ≤ 50 (n = 70) . | > 50 (n = 162) . | Total (n = 241) . | ≤ 30 (n = 19) . | 31-50 (n = 60) . | ≤ 50 (n = 79) . | > 50 (n = 162) . | |
| Response-evaluable, n | 221 | 11 | 52 | 63 | 158 | 235 | 19 | 58 | 77 | 158 |
| Response rate, % | 93.2 | 81.8a | 96.2b | 93.7c | 93d | 83 | 68.4 | 81 | 77.9 | 85.4e |
| CR rate, % | 39.4 | 36.4f | 42.3g | 41.3h | 38.6i | 25.5 | 15.8 | 25.9 | 23.4 | 26.6j |
| Median time to first response, mo | 1.4 | 1.2k | 1.4l | 1.4m | 1.4n | 1.4 | 1.4 | 1.4 | 1.4 | 1.4o |
| Median duration of response, mo | NR | 19.8p | NRq | NRr | NRs | 27.9 | 20 | 22 | 21.8 | 28.6t |
| Median PFS, mo | NR | 20.9 | NR | NR | NR | 24.6 | 22.5 | 24.2 | 24.2 | 25.1 |
| 1-year PFS, % | 93 | 80 | 96 | 96 | 92 | 87 | 83 | 89 | 87 | 87 |
| 2-year PFS, % | 69 | 40 | 73 | 69 | 69 | 55 | 46 | 57 | 54 | 56 |
| Median OS, mo | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 1-year OS, % | 92.3 | 75.2 | 94.2 | 90.7 | 93 | 93.8 | 88.9 | 93.1 | 92.1 | 95.3 |
| 2-year OS, % | 88 | 60.2 | 89.6 | 84.2 | 89.6 | 89.3 | 83.3 | 88.7 | 87.3 | 90.3 |
PFS and OS were analyzed in all 473 patients.
CR indicates complete response; NR, not reached; OS, overall survival; PFS, progression-free survival; VMP, bortezomib-melphalan-prednisone; and VMPT-VT, bortezomib-melphalan-prednisone-thalidomide followed by bortezomib-thalidomide maintenance.
P = .3 for the comparison with VMP patients with eGFR ≤ 30;
P = .026 for the comparison with VMP patients with eGFR 31-50;
P = .015 for the comparison with VMP patients with eGFR ≤ 50;
P = .22 for the comparison with VMPT-VT patients with eGFR31-50 and VMPT-VT patients with eGFR ≤ 30;
P = .16 for the comparison with VMP patients with eGFR 31-50 and VMP patients with eGFR ≤ 30;
P = .2 for the comparison with VMP patients with eGFR ≤ 30;
P = .07 for the comparison with VMP patients with eGFR 31-50;
P = .025 for the comparison with VMP patients with eGFR ≤ 50;
P = .87 for the comparison with VMPT-VT patients with eGFR31–50 and VMPT-VT patients with eGFR ≤ 30;
P = .59 for the comparison with VMP patients with eGFR 31-50 and VMP patients with eGFR ≤ 30;
P = .62 for the comparison with VMP patients with eGFR ≤ 30;
P = .61 for the comparison with VMP patients with eGFR 31-50;
P = .51 for the comparison with VMP patients with eGFR ≤ 50;
P = .89 for the comparison with VMPT-VT patients with eGFR 31-50 and VMPT-VT patients with eGFR ≤ 30;
P = .82 for the comparison with VMP patients with eGFR 31-50 and VMP patients with eGFR ≤ 30;
P = .18 for the comparison with VMP patients with eGFR ≤ 30;
P = .47 for the comparison with VMP patients with eGFR 31-50;
P = .83 for the comparison with VMP patients with eGFR ≤ 50;
P = .41 for the comparison with VMPT-VT patients with eGFR 31-50 and VMPT-VT patients with eGFR < 30; and tP = .57 for the comparison with VMP patients with eGFR 31-50 and VMP patients with eGFR < 30.
Although a significantly longer DOR was observed in patients treated in the VMPT-VT arm (HR 1.5, 95% confidence interval [CI] 1.1-2.1, P = .033), this difference was no longer statistically significant when cases were categorized with respect to both treatment arm and renal function (Table 2). Furthermore, within the VMPT-VT and VMP arms RI did not influence the DOR.
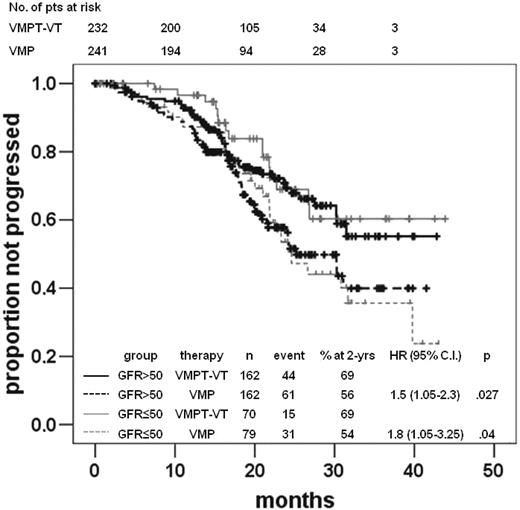
A total of 152 the 473 (32%) patients progressed; the median PFS for the entire population was 31 months. Patients treated with VMPT-VT showed a better PFS than those with VMP (HR 1.6, 95% CI 1.2-2.3, P = .003), even when patients were regrouped into those without (HR 1.5, 95% CI 1.1-2.3, P = .029) or with RI (HR 1.9, 95% CI 1.1-3.5, P = .043; Figure 1). However, this advantage in favor of VMPT-VT was only observed in patients with moderate RI (HR 2.1, 95% CI 1.1-4.3, P = .033), not in those with severe RI (HR 0.9, 95% CI 0.2-3.6, P = .9). No difference was observed between the 2 arms across renal cohorts of patients.
Time to progression according to therapy arms and renal function. Time to progression in the VMPT-VT and VMP arms in patients with normal renal function (eGFR ≥ 50 mL/min) or RI (eGFR < 50 mL/min).
Time to progression according to therapy arms and renal function. Time to progression in the VMPT-VT and VMP arms in patients with normal renal function (eGFR ≥ 50 mL/min) or RI (eGFR < 50 mL/min).
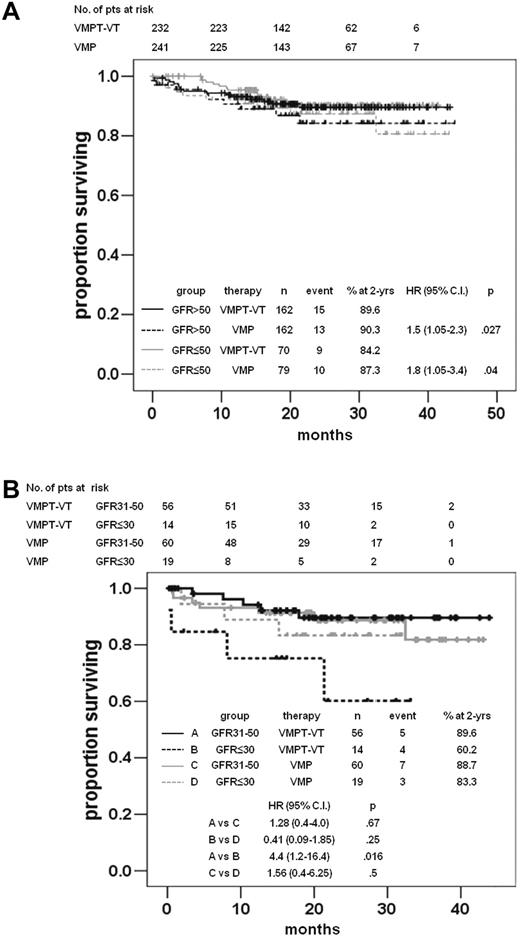
After a median follow-up of 21.6 months, 40 patients died, and the 1-year and 2-year OS rates were similar in the 2 treatment arms (Table 2). No difference in terms of OS was observed when cases were clustered with respect to both treatment arm and renal function (Figure 2A). Notably, within the VMPT-VT arm, a statistically significant shorter OS was observed in patients with severe than those with moderate RI (2-year OS probability: VMPT-VT patients with eGFR 31-50 mL/min vs VMPT-VT patients with eGFR ≤ 30 mL/min, 89.6% vs 60.2% respectively; P = .016; Figure 2B). In contrast, no differences were observed within the VMP arm (2-year OS probability: VMP patients with eGFR 31-50 mL/min vs VMP patients with eGFR ≤ 30 mL/min, 88.7% vs 83.3%, respectively, P = .5; Figure 2B). No statistically different OS was observed between the 2 arms among patients with severe RI (2-year OS probability: VMPT-VT patients with eGFR ≤ 30 mL/min vs VMP patients with eGFR ≤ 30 mL/min, 60.2% vs 83.3% respectively, P = .25).
OS according to therapy arms and renal function. (A) OS in the VMPT-VT and VMP arms in patients with normal renal function (eGFR ≥ 50 mL/min) or RI (eGFR < 50 mL/min). (B) OS in the VMPT-VT and VMP arms in patients with moderate RI (eGFR 31-50 mL/min) and severe RI (eGFR < 30 mL/min).
OS according to therapy arms and renal function. (A) OS in the VMPT-VT and VMP arms in patients with normal renal function (eGFR ≥ 50 mL/min) or RI (eGFR < 50 mL/min). (B) OS in the VMPT-VT and VMP arms in patients with moderate RI (eGFR 31-50 mL/min) and severe RI (eGFR < 30 mL/min).
Reversal of RI
Forty-seven of 140 (33.6%) cases with RI recovered their renal function. The rates of RI reversal were 16 of 63 (25.4%) for VMPT-VT compared with 31 of 77 (40.3%) on the VMP arm (OR = 1.8, P = .092). Notably, for those patients with baseline eGFR ≤ 30 mL/min, none recovered from RI in the VMPT-VT arm, whereas 2 of 19 achieved a normalization of renal function in the VMP arm (P = .25). On the basis of the criteria of Ludwig et al,31 25.4% (16 of 63) and 40.3% (31 of 77) of patients on VMPT-VT and VMP arms had a renal CR. None of the patients of either arm had a partial renal response, whereas 7 and 8 patients, respectively, on the VMPT-VT and VMP arms had a minimal renal response.
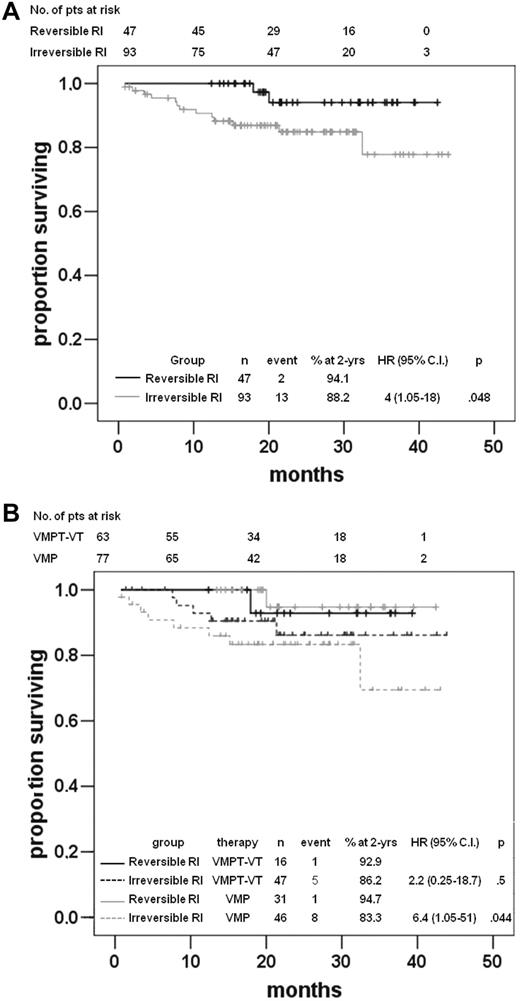
Although there was a trend toward a greater rate of renal improvement in the VMP arm, times to reversal of RI were not significantly different in patients treated with VMPT-VT versus VMP (Figure 3). Among patients achieving RI reversal, median time to reversal was 2.3 months (range, 0.5-12 months) and 2.2 months (range, 0.4-10 months) for VMPT-VT and VMP arms, respectively. Among several factors potentially affecting the rate of RI reversal other than the trial arm (ie, bortezomib once vs twice weekly, age, β2-microglobulin, albumin and LDH serum levels, cytogenetic risk, response to therapy, performance status, the severity of RI, sex), only male sex (OR 0.4, P = .016) and eGFR ≥ 30 mL/min (OR 0.1, P = .004) significantly predicted RI reversal (Table 3). By logistic multivariate analysis, male sex (RR 0.4, 95% CI 0.2-085, P = .022) and moderate RI (RR 0.1, 95% CI 0.02-0.48 P = .003) significantly predicted renal function recovery (Table 3). The treatment arm did not influence the RI reversal by univariate and multivariate analyses. Patients achieving a reversal in RI did not show any statistically different ORR, time to first response, DOR, and PFS than those who did not, whereas OS was significantly shorter for patients who did not recover from RI (P = .048; Figure 4A). Notably, 0 of 4 and 5 of 17 cases (P = .53) with RI who did not respond to VMPT-VT or VMP demonstrated a reversal or RI. Within the VMPT-VT and VMP arms median time to first response, as well as DOR and PFS were not influenced by RI reversal. Interestingly, OS curves were significantly different between patients achieving a renal response or those who did not, only in the VMP arm (Figure 4B).
Time to reversal of renal recovery according to therapy. Time to reversal of renal recovery in the VMPT-VT and VMP arms in patients with RI (eGFR < 50 mL/min).
Time to reversal of renal recovery according to therapy. Time to reversal of renal recovery in the VMPT-VT and VMP arms in patients with RI (eGFR < 50 mL/min).
Factors affecting rate of reversal of renal impairment by univariate and multivariate analysis
| Factor . | Reversal rate, % . | OR (95% CI) . | P (uni) . | P (multi) . |
|---|---|---|---|---|
| Age, y | ||||
| < 75 | 35.8 | 0.7 (0.37-1.58) | .46 | |
| ≥ 75 | 29.8 | |||
| Sex | ||||
| Male | 44.8 | 0.4 (0.2-0.85) | .016 | .022 |
| Female | 25 | |||
| Karnofsky performance status | ||||
| > 70 | 32.2 | 1.15 (0.55-2.41) | .70 | |
| ≤ 70 | 35.4 | |||
| e-GFR, mL/min | ||||
| ≥ 30 | 40.4 | 0.1 (0.02-0.48) | .004 | .003 |
| < 30 | 6.9 | |||
| β2-microglobulin, mg/dL | ||||
| < 3.5 | 41.7 | 0.52 (0.2-1.34) | .18 | |
| ≥ 3.5 | 27.3 | |||
| Albumin, g/dL | ||||
| ≥ 3.5 | 33.3 | 0.67 (0.28-1.56) | .35 | |
| < 3.5 | 25 | |||
| LDH serum levels | ||||
| Normal | 32.7 | 1.29 (0.39-4.25) | .67 | |
| Abnormal | 38.5 | |||
| Cytogenetic risk | ||||
| Standard | 29.5 | 1.33 (0.53-3.31) | .54 | |
| High | 35.7 | |||
| Response (≥ PR) | ||||
| Yes | 35.3 | 0.57 (0.19-1.67) | .3 | |
| No | 23.8 | |||
| Arm | ||||
| VMPT-VT | 25.8 | 1.87 (0.9-3.90) | .09 | .06 |
| VMP | 40.3 | |||
| Bortezomib schedule | ||||
| Once weekly | 30.5 | 1.68 (0.75-3.76) | .2 | |
| Twice weekly | 42.4 |
| Factor . | Reversal rate, % . | OR (95% CI) . | P (uni) . | P (multi) . |
|---|---|---|---|---|
| Age, y | ||||
| < 75 | 35.8 | 0.7 (0.37-1.58) | .46 | |
| ≥ 75 | 29.8 | |||
| Sex | ||||
| Male | 44.8 | 0.4 (0.2-0.85) | .016 | .022 |
| Female | 25 | |||
| Karnofsky performance status | ||||
| > 70 | 32.2 | 1.15 (0.55-2.41) | .70 | |
| ≤ 70 | 35.4 | |||
| e-GFR, mL/min | ||||
| ≥ 30 | 40.4 | 0.1 (0.02-0.48) | .004 | .003 |
| < 30 | 6.9 | |||
| β2-microglobulin, mg/dL | ||||
| < 3.5 | 41.7 | 0.52 (0.2-1.34) | .18 | |
| ≥ 3.5 | 27.3 | |||
| Albumin, g/dL | ||||
| ≥ 3.5 | 33.3 | 0.67 (0.28-1.56) | .35 | |
| < 3.5 | 25 | |||
| LDH serum levels | ||||
| Normal | 32.7 | 1.29 (0.39-4.25) | .67 | |
| Abnormal | 38.5 | |||
| Cytogenetic risk | ||||
| Standard | 29.5 | 1.33 (0.53-3.31) | .54 | |
| High | 35.7 | |||
| Response (≥ PR) | ||||
| Yes | 35.3 | 0.57 (0.19-1.67) | .3 | |
| No | 23.8 | |||
| Arm | ||||
| VMPT-VT | 25.8 | 1.87 (0.9-3.90) | .09 | .06 |
| VMP | 40.3 | |||
| Bortezomib schedule | ||||
| Once weekly | 30.5 | 1.68 (0.75-3.76) | .2 | |
| Twice weekly | 42.4 |
e-GFR indicates estimated glomerular filtration rate; high-risk cytogenetic profile, presence of a t(4;14), t(14,16), or a 17p deletion; PR, partial remission; VMP, bortezomib-melphalan-prednisone; and VMPT-VT, bortezomib-melphalan-prednisone-thalidomide followed by bortezomib-thalidomide maintenance.
OS. (A) OS by reversal of RI. (B) OS in the VMPT-VT and VMP arms by reversal of RI.
OS. (A) OS by reversal of RI. (B) OS in the VMPT-VT and VMP arms by reversal of RI.
Safety
All renal cohorts received a median of 9 treatment cycles, whereas those treated with VMPT having eGFR ≤ 30 mL/min received a median of 7.5 cycles. Table 4 lists the overall grade 3/4 AEs during VMPT-VT and VMP induction subdivided by renal cohort. Grade 3/4 hematologic AEs were similar in both groups, but severe neutropenia was more frequent after treatment with VMPT-VT (37.5% vs 27%; P = .014). In both arms, patients with eGFR ≤ 50 mL/min showed a statistically greater rate of grade 3/4 hematologic AEs than patients with eGFR > 50 mL/min (VMPT-VT: anemia, P < .0001; neutropenia, P = .046; thrombocytopenia, P = .006; VMP: anemia, P = .005, thrombocytopenia, P = .001). However, in the VMP arm, neutropenic episodes was similar across patients with different eGFR rates (P = .34).
Grade 3/4 adverse events during induction treatment of patients distributed by VMPT-VT and VMP arms and by renal cohorts
| Adverse events, n (%) . | VMPT-VT arm, estimated glomerular filtration rate . | VMP arm, estimated glomerular filtration rate . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 232) . | ≤ 30 (n = 14) . | 31-50 (n = 56) . | ≤ 50 (n = 70) . | > 50 (n = 162) . | Total (n = 241) . | ≤ 30 (n = 19) . | 31-50 (n = 60) . | ≤ 50 (n = 79) . | > 50 (n = 162) . | |
| Hematologic events | 109 (47) | 9 (64.3) | 33 (58.9) | 42 (60) | 67 (41.4) | 97 (40.2) | 11 (57.9) | 31 (51.7) | 42 (53.2) | 55 (34) |
| Neutropenia | 87 (37.5) | 8 (57.1)* | 25 (44.6) | 33 (47.1) | 54 (33.3) | 65 (27) | 4 (21.1) | 21 (35) | 25 (31.6) | 40 (24.7) |
| Thrombocytopenia | 50 (21.6) | 6 (44.9) | 17 (34.4) | 23 (32.9) | 27 (16.7) | 47 (19.5) | 6 (31.6) | 19 (31.7) | 25 (31.6) | 22 (13.6) |
| Anemia | 25 (10.8) | 4 (28.6) | 12 (21.4) | 16 (22.9) | 9 (5.6) | 24 (10) | 6 (31.6) | 8 (13.3) | 14 (17.7) | 10 (6.2) |
| Cardiologic events | 25 (10.8) | 3 (21.4) | 9 (16.1) | 12 (17.1) | 13 (8) | 11 (4.6) | 1 (5.3) | 4 (6.7) | 5 (6.3) | 6 (3.7) |
| Infections | 31 (13.4) | 1 (7.1) | 10 (17.9) | 11 (17.5) | 20 (12.3) | 22 (9.1) | 4 (21) | 6 (10) | 10 (12.7) | 12 (7.4) |
| Gastrointestinal events | 16 (6.9) | 2 (14.3) | 5 (8.9) | 7 (10) | 9 (5.1) | 20 (8.3) | 2 (10.5) | 3 (5) | 5 (6.3) | 15 (9.3) |
| Vascular events | 13 (5.6) | 2 (14.3) | 5 (8.9) | 7 (10) | 6 (3.7) | 5 (2.1) | 0 (0) | 1 (1.7) | 1 (1.3) | 4 (2.5) |
| Systemic events | 16 (6.9) | 2 (14.3) | 6 (10.7) | 8 (11.4) | 8 (4.9) | 8 (3.3) | 2 (10.5) | 3 (5) | 5 (6.3) | 3 (1.9) |
| Dermatologic events | 9 (3.9) | 1 (7.1) | 4 (7.1) | 5 (7.1) | 4 (2.5) | 6 (2.5) | 0 (0) | 1 (1.7) | 1 (1.3) | 5 (3.1) |
| Sensory neuropathy and/or neuralgia | 32 (13.8) | 3 (21.4) | 7 (12.5) | 10 (14.3) | 22 (13.6) | 28 (11.6) | 2 (10.5) | 4 (6.7) | 6 (7.6) | 22 (13.6) |
| Discontinuation attributable to adverse events | 51 (22) | 4 (28.6) | 14 (25) | 18 (25.7) | 33 (20.4) | 34 (14.1) | 4 (21.1) | 6 (10)† | 10 (12.7) | 24 (14.8) |
| Adverse events, n (%) . | VMPT-VT arm, estimated glomerular filtration rate . | VMP arm, estimated glomerular filtration rate . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 232) . | ≤ 30 (n = 14) . | 31-50 (n = 56) . | ≤ 50 (n = 70) . | > 50 (n = 162) . | Total (n = 241) . | ≤ 30 (n = 19) . | 31-50 (n = 60) . | ≤ 50 (n = 79) . | > 50 (n = 162) . | |
| Hematologic events | 109 (47) | 9 (64.3) | 33 (58.9) | 42 (60) | 67 (41.4) | 97 (40.2) | 11 (57.9) | 31 (51.7) | 42 (53.2) | 55 (34) |
| Neutropenia | 87 (37.5) | 8 (57.1)* | 25 (44.6) | 33 (47.1) | 54 (33.3) | 65 (27) | 4 (21.1) | 21 (35) | 25 (31.6) | 40 (24.7) |
| Thrombocytopenia | 50 (21.6) | 6 (44.9) | 17 (34.4) | 23 (32.9) | 27 (16.7) | 47 (19.5) | 6 (31.6) | 19 (31.7) | 25 (31.6) | 22 (13.6) |
| Anemia | 25 (10.8) | 4 (28.6) | 12 (21.4) | 16 (22.9) | 9 (5.6) | 24 (10) | 6 (31.6) | 8 (13.3) | 14 (17.7) | 10 (6.2) |
| Cardiologic events | 25 (10.8) | 3 (21.4) | 9 (16.1) | 12 (17.1) | 13 (8) | 11 (4.6) | 1 (5.3) | 4 (6.7) | 5 (6.3) | 6 (3.7) |
| Infections | 31 (13.4) | 1 (7.1) | 10 (17.9) | 11 (17.5) | 20 (12.3) | 22 (9.1) | 4 (21) | 6 (10) | 10 (12.7) | 12 (7.4) |
| Gastrointestinal events | 16 (6.9) | 2 (14.3) | 5 (8.9) | 7 (10) | 9 (5.1) | 20 (8.3) | 2 (10.5) | 3 (5) | 5 (6.3) | 15 (9.3) |
| Vascular events | 13 (5.6) | 2 (14.3) | 5 (8.9) | 7 (10) | 6 (3.7) | 5 (2.1) | 0 (0) | 1 (1.7) | 1 (1.3) | 4 (2.5) |
| Systemic events | 16 (6.9) | 2 (14.3) | 6 (10.7) | 8 (11.4) | 8 (4.9) | 8 (3.3) | 2 (10.5) | 3 (5) | 5 (6.3) | 3 (1.9) |
| Dermatologic events | 9 (3.9) | 1 (7.1) | 4 (7.1) | 5 (7.1) | 4 (2.5) | 6 (2.5) | 0 (0) | 1 (1.7) | 1 (1.3) | 5 (3.1) |
| Sensory neuropathy and/or neuralgia | 32 (13.8) | 3 (21.4) | 7 (12.5) | 10 (14.3) | 22 (13.6) | 28 (11.6) | 2 (10.5) | 4 (6.7) | 6 (7.6) | 22 (13.6) |
| Discontinuation attributable to adverse events | 51 (22) | 4 (28.6) | 14 (25) | 18 (25.7) | 33 (20.4) | 34 (14.1) | 4 (21.1) | 6 (10)† | 10 (12.7) | 24 (14.8) |
Comparison for all variables reported in the table the following subgroups: VMPT-VT patients with eGFR ≤ 30 vs VMP patients with eGFR ≤ 30; VMPT-VT patients with eGFR ≤ 30 vs VMPT-VT patients with eGFR 31-50, VMPT-VT patients with eGFR 31-50 vs VMP patients with eGFR 31-50, VMP patients with eGFR ≤ 30 vs VMP patients with eGFR 31-50 only differences resulted statistically significant have been reported in the table.
VMP indicates bortezomib-melphalan-prednisone; and VMPT-VT, bortezomib-melphalan-prednisone-thalidomide following by bortezomib-thalidomide maintenance.
P = .033 for the comparison with VMP patients with eGFR < 30.
P = .033 for the comparison with VMPT-VT patients with eGFR 31-50 and P = .21 for the comparison with VMP patients with eGFR ≤ 30.
The rate of grade 3/4 bortezomib-associated neuropathy/neuralgia was independent of therapy arm and RI (P = .4 and P = .39, respectively). Grade 3/4 cardiac complications (10.8% vs 4.6%, P = .011) and thromboembolic events (5.6% vs 0%, P = .045) were more frequent in patients receiving VMPT-VT (Table 4). Only the incidence of cardiac complications was influenced by renal function within the VMPT-VT arm (grade 3/4 cardiac complications in the VMPT-VT arm: patients with normal renal function vs patients with RI 8 vs 17.1%, P = .04; in the VMP arm: patients with normal renal function vs patients with RI 3.7 vs 6.3%, P = .36; grade 3/4 thromboembolic events in the VMPT-VT arm: patients with normal renal function vs patients with RI 3.7 vs 10%, P = .06, in the VMP arm: patients with normal renal function vs patients with RI 2.5 vs 1.3%, P = .54).
The proportion of patients requiring treatment interruption for AEs was greater in the VMPT-VT group (P = .026), whereas within the 2 arms the presence or absence of RI did not affect the discontinuation rate for AEs (VMPT-VT: P = .37; VMP: P = .65).
No statistically different rates of grade 3/4 hematologic and nonhematologic AEs or of treatment discontinuation were observed between the subgroups of patients with moderate or severe RI within the 2 treatment arms (Table 4). The proportion of cases requiring therapy discontinuation for AEs was significantly greater in VMPT-VT patients with moderate RI than in VMP patients with moderate RI (Table 4). A significantly greater rate of grade 3/4 episodes of neutropenia was observed in patients with severe RI treated with VMPT-VT than those with severe RI treated with VMP (Table 4).
Patients achieving RI reversal had a slight improvement of safety profile than those with irreversible impairment (grade 3/4 hematologic AEs ≥ 3: 60.9% vs 54.3%, P = .49), with an expected trend toward a reduction in the rate of anemia in patients with recovery of renal function (10.9% vs 25%, P = .052).
Discussion
Recent data from our phase 3 randomized trial explored the potential synergies of a rational 4-drug combination (VMPT), followed by VT maintenance, with the standard VMP with no maintenance therapy.25 Herein, we report the analysis of the renal subgroups of this trial and assess the activity, tolerability, and impact on RI reversal of VMPT-VT versus VMP.
In our study, the response indicators were significantly better in the VMPT-VT versus VMP arms in patients with normal renal function (eGFR > 50 mL/min) and in those with RI (eGFR ≤ 50 mL/min). In line with our recently published efficacy results,25 the analysis of this renal cohort showed that VMPT-VT is superior to VMP in terms of ORR, CR rates, DOR, and PFS in untreated MM patients ineligible for autologous stem cell transplantation with RI. The overall efficacy of both regimens seems not to be substantially influenced by RI, because ORR, CR rates, DOR, and PFS results overlapped across patients with or without RI within both arms. Moreover, no difference in terms of OS was observed subdividing patients by treatment and renal function. This is a general finding also underscored by the VISTA trial, in which bortezomib-based therapy showed a good efficacy in untreated and relapsed/refractory patients with RI,15-18,32 including those requiring dialysis.15-18,20,21,31-37 In addition, the rapidity of response, an important end point for patients with RI, was not influenced by renal function in both arms.
However, when patients were analyzed according to degree of RI, VMPT-VT remained statistically superior to VMP in terms of ORR and PFS only in the moderate RI subgroup but not in those with severe RI. In this small cohort, differences between VMPT-VT and VMP in terms of ORR, CR rates, PFS, and OS did not reach statistical significance, probably because of limited patient numbers. Notably, within the VMPT-VT arm, patients with severe RI showed a shorter OS than the other renal subgroups, probably reflecting coexistence of adverse factors. In particular, VMPT-VT cases with severe RI received fewer cycles of chemotherapy than others (7.5 vs 9 cycles) and had a greater rate of unfavorable cytogenetics. Moreover, none of these achieved a RI reversal. The small number of cases in the severe RI subgroups did not allow us to definitively conclude whether the addition of thalidomide to VMP worsens outcome in patients with severe RI.
The safety profile of both arms appeared only somewhat influenced by RI. Although in patients with eGFR ≤ 50 mL/min the rates of grade 3/4 hematologic AEs appeared statistically greater compared with patients with greater eGFR, the median number of cycles administered and the rate of discontinuation to AE were similar between patients with normal renal function and RI within the 2 arms. This finding is in line with other reports in which renal function did not influence the safety of bortezomib therapy.15-17,32 Similarly to the VISTA trial,19 the VMP safety profile appeared to be moderately affected by RI, whereas in contrast, we observed slightly less hematologic AEs. This phenomenon could be because of the weekly administration of bortezomib adopted in roughly 75% of our patients as a result of the amended protocol.
In the VMPT-VT and VMP arms, the rates of RI reversal were 25.4% and 40.3%, respectively; notably, for those patients with eGFR ≤ 30 mL/min, the rate of RI reversal was 0% in the VMPT-VT arm and 11% in the VMP arm. Data of RI reversal in the VMP arm are in line with those showed in the VISTA (Vascular Inflammation Suppression to Treat Acute Coronary Syndrome for 16 Weeks) trial (40%).19 In other studies of bortezomib-based regimens investigators have also reported similar notable levels of RI reversal.18,20-22,33,34,36,37 In contrast to the VISTA trial,19 in which advanced age together with an abnormal renal function (eGFR < 30 mL/min) represented negative factors for RI recovery, in our study logistic multivariate analysis indicated male sex, but not age, as predictors of renal function recovery, whereas the moderate RI was confirmed as an independent prognostic factor also in our series. However, it is interesting to note that both the rate of and the time to RI reversal did not differ significantly in the 2 arms. These findings indicate that the addition of thalidomide does not improve the results achieved with VMP in patients with RI, in terms of recovery of renal function. Nevertheless, Kastritis et al24 observed a reversal of RI with thalidomide and high-dose dexamethasone, with or without bortezomib, in 80% of previously untreated patients. However, the latter study clearly demonstrated the crucial role of high-dose dexamethasone for obtaining high rates of renal function reversal, when combined with either the older or the newer drugs.24
Interestingly, as reported in the VISTA trial19 and in other studies,3,34 only a few cases in the VMP arm achieved renal response without a MM response, suggesting that even minor reductions in tumor burden may be associated with reversal of RI; one reason may be that bortezomib has a direct effect on renal function.5
Finally, cases in which a reversal of RI occurred experienced a slight improvement of safety profile compared with those with irreversible impairment. OS was significantly shorter for those patients who did not recover from RI, and more interestingly, OS curves diverged significantly in the VMP but not in the VMPT-VT arm. This difference in OS may reflect more advanced disease in patients with RI that seems to be rescued by the addition of thalidomide to the VMP treatment regimen.
In conclusion, this cohort analysis reflects the overall efficacy of VMPT-VT resulting from our recently published phase 3 randomized trial; VMPT-VT remains superior to VMP for patients with moderate RI. As can been deduced, VMPT-VT failed to outperformed VMP in patients with severe RI. Nonetheless, drawing definitive conclusions regarding this combination schedule in severe RI patients is precluded because of the fact that the protocol was not designed to demonstrate this issue because patients with sCr levels ≥ 2.5 mg/dL were excluded from the study and, consequently, the severe RI cases included in this analysis are limited. Finally, it seems that VMPT-VT patients with severe RI show a greater, although not significant, rate of high-risk cytogenetics.
The safety of both therapy arms is not substantially influenced by the degree of RI in our analysis, but a greater rate of severe hematologic AEs was observed presumably because of the effect of melphalan. Moreover, the addition of thalidomide to VMP did not have an impact on the reversal of RI. Thus, we can conclude that the use of VMPT-VT as a treatment can be justified outside of a clinical trial in cases with normal or moderately abnormal renal function, with bortezomib-based therapy overall showing a favorable effect in patients with both moderate and severe RI.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Brigida Gulino for precious secretarial assistance.
This study was supported by Fondazione “Amelia Scorza” Onlus, Cosenza, Italy.
Authorship
Contribution: F.M., M.G., M.B., and A.P. conceived and designed the study; C.M., D.R., F.D., S.B., R.R., M.O., F.P., C.N., M.T.P., G.B., I.V, T.G., M.G., R.M., L.B., V.M., P.M., N.C., I.M., C.M., and M.C. provided study materials or patients; F.M., M.G., and A.P. analyzed and interpreted the data; F.M., M.G., M.B., and A.P. wrote the manuscript; and F.M., M.G., C.M., D.R., F.D., S.B., R.R., M.O., F.P., C.N., M.T.P., G.B., I.V., T.G., M.G., R.M., L.B., V.M., P.M., N.C., I.M., C.M., M.C., M.B., and A.P. approved the manuscript.
Conflict-of-interest disclosure: A.P. is a consultant for Celgene and Janssen-Cilag; M.C. for Janssen-Cilag and Millennium Pharmaceuticals; S.B. for Merck Sharp & Dohme; and M.B. for Celgene and Janssen-Cilag. A.P. has received honoraria from Celgene, Janssen-Cilag; M.C. from Janssen-Cilag, Novartis; S.B. from Celgene, Janssen-Cilag, and Novartis; F.P. from Celgene, Janssen-Cilag, and Schering-Plough; R.R. from Novartis, Celgene, and Janssen-Cilag; L.B. from Celgene and Janssen-Cilag; M.O. from Celgene and Janssen-Cilag; M.B. from Celgene and Janssen-Cilag; P.M. from Celgene and Janssen-Cilag; and M.T. from Petrucci, Celgene, and Janssen-Cilag. R.M. received research funding from Celgene; and M.B. from Celgene and Janssen-Cilag.
Correspondence: Fortunato Morabito, Unità Operativa Complessa di Ematologia, Dipartimento Oncoematologico, Azienda Ospedaliera di Cosenza, Viale della Repubblica, 87100 Cosenza, Italy; e-mail: fortunato_morabito@tin.it.