Abstract
IL-36α (IL-1F6), IL-36β (IL-1F8), and IL-36γ (IL-1F9) are members of the IL-1 family of cytokines. These cytokines bind to IL-36R (IL-1Rrp2) and IL-1RAcP, activating similar intracellular signals as IL-1, whereas IL-36Ra (IL-1F5) acts as an IL-36R antagonist (IL-36Ra). In this study, we show that both murine bone marrow-derived dendritic cells (BMDCs) and CD4+ T lymphocytes constitutively express IL-36R and respond to IL-36α, IL-36β, and IL-36γ. IL-36 induced the production of proinflammatory cytokines, including IL-12, IL-1β, IL-6, TNF-α, and IL-23 by BMDCs with a more potent stimulatory effect than that of other IL-1 cytokines. In addition, IL-36β enhanced the expression of CD80, CD86, and MHC class II by BMDCs. IL-36 also induced the production of IFN-γ, IL-4, and IL-17 by CD4+ T cells and cultured splenocytes. These stimulatory effects were antagonized by IL-36Ra when used in 100- to 1000-fold molar excess. The immunization of mice with IL-36β significantly and specifically promoted Th1 responses. Our data thus indicate a critical role of IL-36R ligands in the interface between innate and adaptive immunity, leading to the stimulation of T helper responses.
Introduction
The IL-1 family of cytokines is composed of 11 different ligands, namely, IL-1α (also termed IL-1F1), IL-1β (IL-1F2), IL-1 receptor antagonist (IL-1Ra or IL-1F3), IL-18 (IL-1F4), IL-1F5 to IL-1F10, and IL-1F11 (or IL-33). IL-1α and IL-1β are known to induce proinflammatory activities on binding to type I IL-1 receptor (IL-1RI) and recruitment of the common coreceptor IL-1 receptor accessory protein (IL-1RAcP), whereas IL-1Ra acts as a competitive inhibitor of IL-1 binding to IL-1RI, thus exerting anti-inflammatory activity.1 Numerous studies reported that IL-18 is a proinflammatory cytokine that is clearly more than an inducer of IFN-γ, whereas IL-33 was described as an immunoregulatory cytokine involved in particular in the control of Th2 responses.2 Ten years ago, new members of the IL-1 family, including IL-1F5, IL-1F6, IL-1F8, and IL-1F9, were identified through searches in DNA databases for homologs of IL-1.3,4 In humans and mice, all the genes encoding these cytokines map to less than 300 kb of chromosome 2q, where they are flanked by the IL1A, IL1B, and IL1RN genes.4-11 IL-1F6, IL-1F8, and IL-1F9 share 21% to 37% amino acid sequence homology with IL-1 and IL-1Ra, whereas IL-1F5 displays 52% amino acid sequence homology with IL-1Ra, suggesting that IL-1F5 might represent an endogenous receptor antagonist.6,9
IL-1F6, IL-1F8, and IL-1F9 bind to IL-1Rrp2, a receptor of the IL-1R family, and use IL-1RAcP as a coreceptor to stimulate intracellular signals similar to those induced by IL-1,12 whereas IL-1F5 was shown to inhibit IL-1F9–induced NF-κB activation in Jurkat T cells that overexpress IL-1Rrp2.13 Like IL-1β, all these IL-1 homologs lack a leader peptide and cannot be released through the conventional secretory pathway, although studies suggest that release of IL-1Rrp2 agonists may be controlled by mechanisms different from those regulating IL-1β secretion.14 To acknowledge the specific biologic effects of these cytokines and to recognize that they all bind to the same receptor, it has recently been proposed to amend the nomenclature of IL-1 homologs. Thus, IL-1Rrp2 is now termed IL-36R and its ligands are named IL-36α (IL-1F6), IL-36β (IL-1F8), and IL-36γ (IL-1F9). In addition, IL-1F5, which has been shown to exert receptor antagonist activities, has been renamed IL-36Ra.15
Currently, little is known regarding the expression pattern and the biologic function of IL-36α, IL-36β, and IL-36γ. Messenger RNAs for these cytokines are highly expressed in only a few tissues, particularly in internal epithelial tissues, which are exposed to pathogens and in skin.6,12,13 Interestingly, expression of IL-36Ra and IL-36α is significantly up-regulated in IL-1β/TNF-α–stimulated human keratinocytes, and IL-36Ra and IL-36γ mRNA are highly increased in lesional psoriasis skin.13 Moreover, IL-36γ protein production is enhanced in human keratinocytes after TNF-α and IFN-γ stimulation.6 Elevated IL-36α mRNA and protein expression was reported also in chronic kidney disease.16 Finally, in previous studies, we have shown that IL-36β mRNA is expressed in human rheumatoid synovial tissues and that synovial fibroblasts express IL-36R.17
The in vivo effects of IL-36R ligands remain also poorly characterized. Transgenic mice overexpressing IL-36α in keratinocytes exhibit inflammatory skin lesions sharing some features with psoriasis. This phenotype was more severe when transgenic mice were crossed with IL-36Ra–deficient mice, supporting a regulatory function of IL-36Ra in vivo.18 The inflammatory skin condition in keratinocyte-specific IL-36α transgenic is even more similar to human psoriasis if the mice are treated with12-O-tetradecanoylphorbol 13-acetate, resembling the human disease histologically, molecularly, and in its response to therapeutics. Moreover, human psoriatic lesional skin transplanted onto immunodeficient mice is normalized when the mice are treated with anti–IL-36R antibody, arguing that the IL-36 axis is required to maintain the lesional phenotype in human psoriatic skin. Taken together, these data indicate that IL-36R ligands, including IL-36α, IL-36β, and IL-36γ, exert proinflammatory effects in vitro and in vivo and that IL-36Ra acts as a natural antagonist, thus mimicking the IL-1/IL-1Ra system. However, the role of endogenous IL-36R and IL-36R ligands is still unclear.
In the results included herein, we show that IL-36R is expressed in dendritic cells (DCs) and CD4+ T cells and that IL-36R agonist ligands are able to stimulate the production of several cytokines by DCs, whereas IL-36Ra exerts an inhibitory effect. In addition, we show that IL-36R agonist ligands are potent regulators of T-cell responses in vitro and in vivo. These results demonstrate, for the first time, a critical role for these IL-1 homologs in the stimulation of innate and adaptive immune responses.
Methods
Mice
Wild-type (WT) C57BL/6J mice were obtained from Janvier. IL-36R and IL-36Ra–deficient mice (IL-136R−/−, IL-36Ra−/−)18 were backcrossed 9 and 7 times, respectively, in a pure C57BL/6J genetic background by using a marker-assisted selection protocol approach, also termed “speed congenics.”19 All mice were maintained under conventional conditions in the animal facility of the Geneva University School of Medicine, and water and food were provided ad libitum. Animal studies were approved by the Animal Ethics Committee of the Geneva Veterinarian Office and were performed according to the appropriate codes of practice.
Biologic reagents
Media used for mouse primary cell isolation and culture were obtained from Invitrogen. Recombinant mouse IL-33 was provided by Alexis Corp. Recombinant human IL-1β and recombinant mouse IL-18 were purchased from R&D Systems, and purified lipopolysaccharide (LPS) from Fluka (Escherichia coli 055:B5). Murine IL-36Ra, IL-36α, IL-36β, and IL-36γ were produced at Amgen as N-terminal truncation variants that have considerably greater biologic activity than the full-length cytokines (Towne et al, manuscript submitted). Concanavalin A was purchased from GE Healthcare.
Generation, culture, and stimulation of BMDCs, bone marrow macrophages and bone marrow neutrophils
Bone marrow-derived dendritic cells (BMDCs) were obtained from 7- to 12-week-old WT, IL-36R −/− or IL-36Ra −/− C57BL/6 mice. BMDCs were cultured as previously described,20 with slight modifications. Briefly, BM cells were flushed out of the cavities of femur and tibia with RPMI 1640. Adherent and nonadherent cells were separated after incubation for 30 minutes on a 10-cm dish. Nonadherent cells were cultured for 10 days in RPMI 1640 supplemented with 10% FCS, 1% penicillin/streptomycin, and 50μM β-mercaptoethanol. Recombinant murine granulocyte macrophage colony-stimulating factor (rmGM-CSF, 20 ng/mL; Immuno Tools) was added to cell cultures every 2 days. At day 10 of culture, adherent cells were harvested. The percentage of CD11c+ CD11b+ cells was monitored by FACScan and was usually approximately 75%. The remaining CD11c− cells represented mostly bone marrow–derived macrophages. BMDCs (2.5 × 105 cells/well) were seeded into 48-well plates and cultured with or without IL-36Ra, IL-36α, IL-36β, IL-36γ, IL-1β, or LPS at indicated concentrations for 72 hours. Cell supernatants were then harvested and cytokine levels determined by sandwich ELISA or Multiplex assays.
Bone marrow macrophage precursors were isolated from femur and tibia cavities as described in the previous paragraph. Adherent cells were cultured for 6 days in DMEM supplemented with 20% FCS, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 2mM L-glutamine, in the presence of macrophage colony stimulating factor (M-CSF). At day 6 of culture, adherent cells were harvested and used for subsequent experiments as indicated. The percentage of CD11b+ cells was monitored by FACScan and was usually approximately 90%.
Bone marrow neutrophils were isolated from femur and tibia cavities as described in the previous paragraph. Cells were cultured for 9 days in DMEM-Glutamax supplemented with 1× nonessential amino acids, 1mM of sodium pyruvate, 50μM β-mercaptoethanol, and 20% horse serum, in the presence of recombinant murine granulocyte colony stimulating factor (G-CSF, 5 ng/mL, PeproTech). At day 9 of culture, cells were harvested and used for subsequent experiments as indicated. The percentage of Gr-1+ CD11b+ cells was monitored by FACScan and was usually approximately 70%.
T-cell purification, culture, and stimulation
Total CD4+ T cells were purified from spleen by negative selection using immunomagnetic beads according to the manufacturer's protocol (CD4+ T Cell Isolation Kit II Miltenyi Biotec) and passed through a magnetic cell sorting column (Miltenyi Biotec). The percentage of CD4+ cells was monitored by FACScan and was usually 90%. Total CD4+ T cells (3 × 104 cells/well) were activated with plate-bound anti–mouse CD3/CD28 antibodies (0.5 μg/mL/0.5 μg/mL, BD Biosciences PharMingen) in flat-bottom 96-well plates in the presence of IL-36Ra, IL-136α, IL-36β, IL-36γ, and IL-1β (100 ng/mL). After 3 days of stimulation, cell supernatants were collected and cytokines (IFNγ, IL-4, and IL-17) levels were measured by ELISA.
In vitro differentiation of Th1, Th2, and Th17 cells
Stimulation and in vitro polarization of naive T cells are described in detail in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Immunization of mice
Eight- to 10-week-old male WT and IL-36R−/− mice were immunized by intradermal injections at the base of the tail. On day 0, mice were injected with 100 μL of methylated bovine serum albumin (mBSA, Sigma-Aldrich, 2 mg/mL) emulsified in complete Freund adjuvant (CFA, Axon Lab AG), 100 μL of IL-36β (2 μg/mouse) mixed with mBSA (2 mg/mL), or 100 μL mBSA alone (2 mg/mL) in PBS 1 time. Immunization of mice with mBSA/Freund adjuvant was repeated on day 14 in incomplete Freund adjuvant (Chemie Brunschwig AG), whereas mice immunized with mBSA/IL-36β or mBSA/PBS were injected again on days 7 and 14. All the mice were killed at day 21. Cells were isolated from inguinal lymph nodes and restimulated ex vivo with 10 μg/mL of mBSA to determine the production of cytokines (IFN-γ, IL-4, and IL-17). Serum was also collected to measure the levels of anti-mBSA total IgG by ELISA as previously described,21 with slight modifications. Briefly, plates were coated with 100 μL of mBSA (5 μg/mL) overnight at 4°C. The plates were blocked with 1% gelatin (Sigma-Aldrich) in PBS at room temperature for 1 hour. After washing, 100 μL of serum samples was added at various dilutions in 1% gelatin and incubated 2 hours at room temperature. The plates were then washed, and anti–mouse IgG-specific streptavidin-HRP–conjugated antibodies (1 of 5000) were incubated at room temperature for 1 hour. Signal was then detected using substrate Reagent (R&D Systems) at an optical density of 450 nm.
RNA extraction and quantitative real-time PCR
Total RNA extraction, reverse transcription, and quantitative PCR are described in detail in supplemental Methods.
TaqMan low density array
Analysis of cytokines, chemokines, growth factors, and other inflammatory mediators mRNA levels is described in detail in supplemental Methods.
Measurement of cytokine levels
IL-6, IFN-γ, IL-17, and IL-4 levels were determined using ELISA DuoSet kits from R&D Systems. The concentrations of multiple cytokines were determined by a Bio-Plex Pro Mouse Cytokine 32-plex assay (Bio-Rad catalog no. M60009RDPD for 23-plex and catalog no. MD000000EL for 9-plex, Life Science Group) according to the standard protocols provided by the manufacturer. Values obtained were analyzed on the GraphPad Prism Version 5 program.
Flow cytometric analysis
Fluorochrome-labeled mAbs specific for CD11c (HL3), CD11b (M1/70), CD4 (RM4-5), CD62L (MEL-14), CD44 (IM7), CD80 (16-10A1), CD86 (GL1), and MHC-II I-Ab (25-9-17) were obtained from BD Biosciences PharMingen. AlexaFluor-647 mouse anti-p38 MAPK (Thr180/Tyr182) Ab was obtained from BD Biosciences. For cell surface staining, cells were preincubated with mAb 2.4G2 (anti-CD16/32) to block Fc receptors and labeled with mAbs in PBS 1 time, 0.2% BSA, 10mM EDTA. Labeled cells were run on a FACSCalibur, and data were analyzed using CellQuest Version 3 software. For intracellular phospho-p38 MAPK analysis, BMDCs were stimulated for 15 minutes with 100 ng/mL IL-36β or LPS. After fixation with 4% paraformaldehyde, cells were permeabilized with BD Perm/Wash buffer (BD Biosciences) and stained with anti-p38 MAPK-AlexaFluor-647 in 1 time PBS 0.2% BSA. Flow cytometry was performed on the FACSCalibur (BD Biosciences).
Statistical analysis
Significant variations were calculated using the unpaired 2-tailed Student t test. P < .05 was considered significant. Results are expressed as the mean ± SD.
Results
Specific stimulatory effect of IL-36 in BMDCs
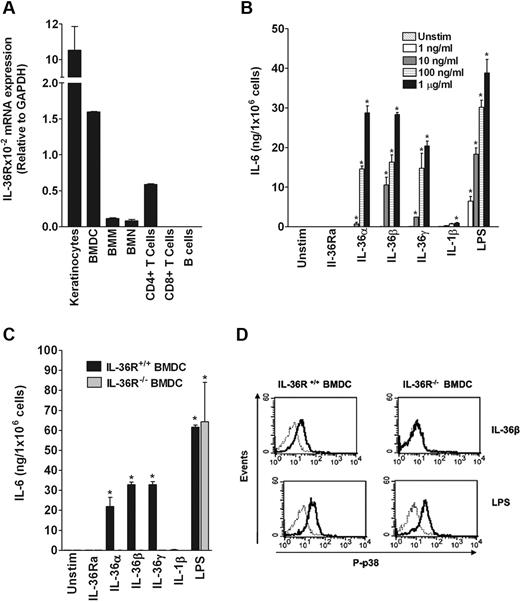
To identify immune cells naturally responding to IL-36α, IL-36β, and IL-36γ, collectively referred to as IL-36, we first examined the expression of mouse IL-36R in several mouse primary cell types and cell lines using quantitative RT-PCR. Among the cells tested, IL-36R mRNA was readily detected in BMDCs, splenic CD4+ T cells, bone marrow–derived macrophages, and bone marrow-derived neutrophils, albeit at a lower level than in keratinocytes, whereas IL-36R mRNA was not detected in CD8+ T cells and B cells (Figure 1A). These results suggested that BMDCs and CD4+ T cells could respond to stimulation with IL-36. As shown in Figure 1B-C, IL-36α, IL-36β, IL-36γ, as well as LPS used as positive control, significantly induced in a dose-dependent manner IL-6 production in BMDCs from WT but not from IL-36R−/− mice. In contrast, IL-1β and IL-33 exerted only a weak stimulatory effect, whereas IL-18 did not significantly stimulate IL-6 production in BMDCs (Figure 1B-C; and data not shown). In addition, IL-36β specifically activated p38 MAPK phosphorylation in WT but not in IL-36R−/− BMDCs (Figure 1D). These results clearly demonstrated that IL-36α, IL-36β, and IL-36γ specifically stimulate BMDCs via signaling through the IL-36R.
Specific effect of IL-36 in inducing IL-6 production in BMDCs. (A) Determination of IL-36 mRNA levels in different cell types. Total mRNA was isolated from primary keratinocytes, BMDCs, bone marrow–derived macrophages, bone marrow–derived neutrophils, CD4+ T cells, CD8+ T cells, and B cells for analyses by quantitative RT-PCR. Results represent IL-36R mRNA expression levels relative to GAPDH. Data are shown from one of 3 independent experiments with similar results. Error bars represent the SD of the mean of triplicates in the same experiment. (B) Dose-dependent effect of IL-36 in BMDCs from WT C57BL/6 mice. BMDCs (2.5 × 105 cells/well) were plated in 48-well plates and cultured in the absence (Unstimulated) or the presence of the indicated concentrations of IL-36Ra, IL-36α, IL-36β, IL-36γ, IL-1β, or LPS for 72 hours. IL-6 levels were measured in culture supernatants by ELISA. Data are shown from one of 3 independent experiments with similar results. Error bars represent the SD of the means of triplicates in the same experiment. *P < .05 (Student t test), IL-36, IL-1β, and LPS significantly differ from unstimulated cells. (C) Stimulatory effects of IL-36α, IL-36β, and IL-36γ are dependent on IL-36R. BMDCs from WT (black bars) or IL-36R−/− (gray bars) C57BL/6 mice were either left unstimulated or stimulated with 1 μg/mL of IL-36α, IL-36β, IL-36γ, or 100 ng/mL of IL-1β or LPS for 72 hours. IL-6 levels were measured in culture supernatants by ELISA. Data are shown from one of 3 independent experiments with similar results. Error bars represent the SD of the means of triplicates in the same experiment. IL-36 and LPS stimulation significantly differs from unstimulated cells (Student t test, P < .05). (D) IL-36β specifically activates the pathway leading to p38 MAPK phosphorylation in BMDCs. Histograms show overlays of phospho-p38 (P-p38) staining in the WT (left panels) or IL-36R−/− BMDCs (right panels) unstimulated (thin gray line) and stimulated (thick black line) with 100 ng/mL of IL-36β or LPS for 15 minutes. Data are shown from 1 of 3 independent experiments with similar results.
Specific effect of IL-36 in inducing IL-6 production in BMDCs. (A) Determination of IL-36 mRNA levels in different cell types. Total mRNA was isolated from primary keratinocytes, BMDCs, bone marrow–derived macrophages, bone marrow–derived neutrophils, CD4+ T cells, CD8+ T cells, and B cells for analyses by quantitative RT-PCR. Results represent IL-36R mRNA expression levels relative to GAPDH. Data are shown from one of 3 independent experiments with similar results. Error bars represent the SD of the mean of triplicates in the same experiment. (B) Dose-dependent effect of IL-36 in BMDCs from WT C57BL/6 mice. BMDCs (2.5 × 105 cells/well) were plated in 48-well plates and cultured in the absence (Unstimulated) or the presence of the indicated concentrations of IL-36Ra, IL-36α, IL-36β, IL-36γ, IL-1β, or LPS for 72 hours. IL-6 levels were measured in culture supernatants by ELISA. Data are shown from one of 3 independent experiments with similar results. Error bars represent the SD of the means of triplicates in the same experiment. *P < .05 (Student t test), IL-36, IL-1β, and LPS significantly differ from unstimulated cells. (C) Stimulatory effects of IL-36α, IL-36β, and IL-36γ are dependent on IL-36R. BMDCs from WT (black bars) or IL-36R−/− (gray bars) C57BL/6 mice were either left unstimulated or stimulated with 1 μg/mL of IL-36α, IL-36β, IL-36γ, or 100 ng/mL of IL-1β or LPS for 72 hours. IL-6 levels were measured in culture supernatants by ELISA. Data are shown from one of 3 independent experiments with similar results. Error bars represent the SD of the means of triplicates in the same experiment. IL-36 and LPS stimulation significantly differs from unstimulated cells (Student t test, P < .05). (D) IL-36β specifically activates the pathway leading to p38 MAPK phosphorylation in BMDCs. Histograms show overlays of phospho-p38 (P-p38) staining in the WT (left panels) or IL-36R−/− BMDCs (right panels) unstimulated (thin gray line) and stimulated (thick black line) with 100 ng/mL of IL-36β or LPS for 15 minutes. Data are shown from 1 of 3 independent experiments with similar results.
Antagonistic effect of IL-36Ra on IL-36α, IL-36β, and IL-36γ in BMDCs
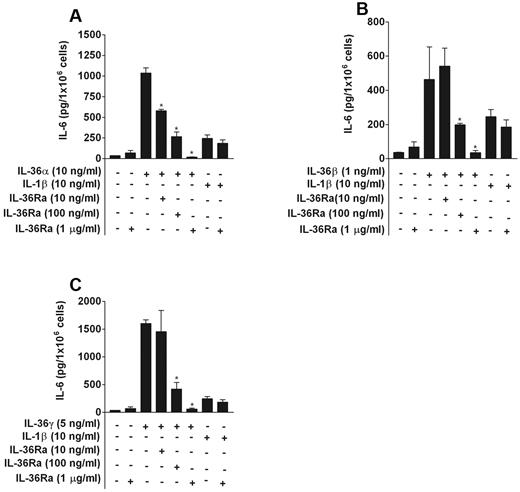
To examine whether IL-36Ra inhibited the stimulatory effects of IL-36α, IL-36β, or IL-36γ, BMDCs were preincubated with increasing concentrations (from 10 ng/mL to 1 μg/mL) of IL-36Ra for 20 minutes before the addition of IL-36α (10 ng/mL), IL-36β (1 ng/mL), IL-36γ (5 ng/mL), or IL-1β (10 ng/mL). As shown in Figure 2, IL-36Ra inhibited the effect of IL-36α, IL-36β, or IL-36γ on IL-6 production, but was devoid of inhibitory effect on IL-1β activity. We observed a significant decrease of IL-6 induction by IL-36α, IL-36β, or IL-36γ ranging from 40% to 95% with the use of increasing concentrations of IL-36Ra up to 1 μg/mL. Thus, IL-36Ra needs to be used in 100- to 1000-fold molar excess to inhibit the effects of IL-36α, IL-36β, or IL-36γ, which is comparable with the inhibitory effect of IL-1Ra on IL-1.22
IL-36Ra acts as a selective inhibitor of IL-36α, IL-36β, and IL-36γ in BMDCs. BMDCs were pretreated or not with 10 ng/mL, 100 ng/mL, or 1 μg/mL IL-36Ra for 20 minutes before the addition of IL-36α (A), IL-36β (B), IL-36γ (C), or IL-1β at the indicated concentrations. After 72 hours, IL-6 levels in cell supernatants were determined by ELISA. *P < .05 versus IL-36α alone (A), IL-36β alone (B), or IL-36γ alone (C). Data are shown from 1 of 3 independent experiments with similar results. Data are mean ± SD of culture triplicates in the same experiment.
IL-36Ra acts as a selective inhibitor of IL-36α, IL-36β, and IL-36γ in BMDCs. BMDCs were pretreated or not with 10 ng/mL, 100 ng/mL, or 1 μg/mL IL-36Ra for 20 minutes before the addition of IL-36α (A), IL-36β (B), IL-36γ (C), or IL-1β at the indicated concentrations. After 72 hours, IL-6 levels in cell supernatants were determined by ELISA. *P < .05 versus IL-36α alone (A), IL-36β alone (B), or IL-36γ alone (C). Data are shown from 1 of 3 independent experiments with similar results. Data are mean ± SD of culture triplicates in the same experiment.
IL-36 induces production of cytokines and expression of MHC and costimulatory molecules in BMDCs
To determine whether IL-36 could stimulate production of other cytokines involved in immune and inflammatory responses, BMDCs were cultured with different cytokines (all used at 100 ng/mL) or LPS as control, for 2 hours and mRNA expression of a panel of cytokines, chemokines, and growth factors was examined by TaqMan low-density array. As illustrated in Table 1, IL-6, IL-12p40, CXCL1, CCL1, IL-12p35, IL-1β, and IL-23p19 mRNA levels were considerably induced (> 60-fold) in BMDCs stimulated by IL-36α, IL-36β, or IL-36γ compared with nonstimulated cells. In addition, GM-CSF, IL-10, CXCL10, and TNF-α mRNA expression was up-regulated more than 10-fold. Finally, cyclooxygenase-2 (COX-2) and nitric oxide synthase 2 (NOS2) mRNA levels were also enhanced by more than 100- and 10-fold, respectively. In contrast, there was low/no induction of IL-3, IL-4, IL-5, or IL-17 mRNA (data not shown). IL-1β exerted merely weak effects compared with those of IL-36α, IL-36β, and IL-36γ, whereas IL-36Ra had no stimulatory activity. Interestingly, IL-36α and IL-36γ mRNA was also expressed in BMDCs. IL-36α mRNA was induced after stimulation (supplemental Figure 1A), whereas IL-36γ mRNA was produced constitutively (supplemental Figure 1B). In contrast, IL-36Ra and IL-36β mRNA levels were not detected in resting or stimulated BMDCs (data not shown). Consistent with the absence of IL-36Ra expression in BMDCs, cells isolated from IL-36Ra−/− mice exhibited similar responses to IL-36α, IL-36β, or IL-36γ as WT BMDCs (supplemental Figure 2).
Effect of IL-36R ligands on mRNA expression of soluble and cell-associated inflammatory mediator by BMDCs
| . | Unstimulated: Ct . | IL-36Ra . | IL-36α . | IL-36β . | IL-36γ . | IL-1β . | LPS . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ct . | Fold . | Ct . | Fold . | Ct . | Fold . | Ct . | Fold . | Ct . | Fold . | Ct . | Fold . | ||
| IL-6 | 29.7 ± 1.8 | 29.9 ± 1.3 | 1.0 | 21.3 ± 0.8 | 218.3 | 21.6 ± 2 | 560 | 21 ± 0.8 | 311.5 | 27.8 ± 1 | 3.3 | 18.5 ± 0.4 | 1216.2 |
| IL-12p40 | 31.1 ± 0.9 | 31.5 ± 0.8 | 0.0 | 25 ± 1.4 | 139.6 | 23.6 ± 1.5 | 426.7 | 22.8 ± 2.1 | 388 | 30 ± 1.3 | 2.5 | 20 ± 1.2 | 1638.8 |
| COX-2 | 29.5 ± 0.2 | 29.8 ± 0.3 | 1 | 22.1 ± 0.5 | 146.5 | 22.6 ± 2.7 | 322 | 21.6 ± 0.6 | 238.7 | 28 ± 0.1 | 2.9 | 20.4 ± 0.4 | 384.5 |
| CXCL1 | 28.5 ± 0.6 | 28.5 ± 0.4 | 1.1 | 20.5 ± 0.5 | 208.9 | 19.9 ± 0.3 | 320.3 | 20.4 ± 0.5 | 239.2 | 25.6 ± 0.3 | 7 | 19.6 ± 0.6 | 297 |
| CCL1 | 33.3 ± 0.9 | 33.1 ± 0.8 | 1.4 | 27.6 ± 2.4 | 68.4 | 27 ± 0.8 | 270.2 | 26 ± 2.2 | 175.4 | 31.4 ± 1.5 | 4.2 | 26.1 ± 1.3 | 107.6 |
| IL-12p35 | 34.3 ± 1.2 | 34.2 ± 0.8 | 1.5 | 27.8 ± 0.9 | 86 | 28 ± 1.7 | 216.5 | 27.4 ± 0.9 | 129.5 | 32.7 ± 1.3 | 4.5 | 27 ± 0.6 | 116.5 |
| IL-1β | 25.1 ± 0.9 | 25.2 ± 0.6 | 1.1 | 17.7 ± 0.6 | 139 | 18.7 ± 2.3 | 211.3 | 17.5 ± 0.6 | 171.6 | 23 ± 0.75 | 5 | 16.9 ± 0.5 | 185 |
| IL-23p19 | 30 ± 1.1 | 30.3 ± 0.9 | 1 | 24 ± 1.2 | 53 | 22.2 ± 0.9 | 207.4 | 23.2 ± 1 | 97.2 | 28.5 ± 0.7 | 2.9 | 22.4 ± 1 | 134.8 |
| GM-CSF | 33 ± 0.7 | 32.6 ± 0.9 | 2 | 27.6 ± 0.8 | 40.6 | 27.8 ± 2 | 111.2 | 26.5 ± 0.7 | 88.2 | 29.5 ± 0.6 | 13.4 | 29 ± 0.5 | 11.8 |
| IL-10 | 34.5 ± 1 | 40 ± 0.0 | 0 | 31.2 ± 0.5 | 16 | 30.4 ± 2.8 | 91 | 29.7 ± 0.7 | 49 | 34.9 ± 0.3 | 2 | 29.5 ± 0.1 | 42 |
| CXCL10 | 28 ± 1.7 | 28 ± 1.5 | 1.5 | 25.8 ± 1.4 | 3.9 | 25.5 ± 4.3 | 47 | 25.5 ± 1.4 | 5 | 27.1 ± 1 | 2 | 18.25 ± 0.7 | 644.3 |
| NOS2 | 30.2 ± 0.2 | 30.6 ± 0.2 | 0.0 | 26.7 ± 0.5 | 9.5 | 26.7 ± 2.5 | 29.4 | 25.8 ± 0.6 | 18.8 | 33.5 ± 1.6 | 3.3 | 23.3 ± 0.4 | 77.3 |
| TNF-α | 23 ± 0.5 | 22.9 ± 0.2 | 1.2 | 18.8 ± 0.45 | 15.3 | 19.7 ± 2.5 | 25.36 | 18.5 ± 0.4 | 19.6 | 21.9 ± 0.3 | 2.2 | 17.3 ± 0.2 | 32.3 |
| CCL3 | 19 ± 0.4 | 18.9 ± 1.2 | 1.5 | 16 ± 0.4 | 6.8 | 17.4 ± 2.4 | 7.8 | 16.1 ± 0.7 | 6.9 | 18.2 ± 0.5 | 1.8 | 16.4 ± 0.5 | 3.8 |
| VCAM-1 | 29.6 ± 1.4 | 29.6 ± 1 | 1.2 | 27.3 ± 1 | 3.8 | 28 ± 2 | 6.5 | 26.9 ± 1 | 5.3 | 29 ± 1 | 1.6 | 25.4 ± 0.5 | 9.8 |
| ICAM-1 | 23.6 ± 0.7 | 23.5 ± 0.2 | 1.2 | 21.6 ± 0.5 | 3 | 23 ± 2.5 | 3.9 | 21.4 ± 0.6 | 3.9 | 23 ± 0.3 | 1.6 | 21.4 ± 0.4 | 3 |
| . | Unstimulated: Ct . | IL-36Ra . | IL-36α . | IL-36β . | IL-36γ . | IL-1β . | LPS . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ct . | Fold . | Ct . | Fold . | Ct . | Fold . | Ct . | Fold . | Ct . | Fold . | Ct . | Fold . | ||
| IL-6 | 29.7 ± 1.8 | 29.9 ± 1.3 | 1.0 | 21.3 ± 0.8 | 218.3 | 21.6 ± 2 | 560 | 21 ± 0.8 | 311.5 | 27.8 ± 1 | 3.3 | 18.5 ± 0.4 | 1216.2 |
| IL-12p40 | 31.1 ± 0.9 | 31.5 ± 0.8 | 0.0 | 25 ± 1.4 | 139.6 | 23.6 ± 1.5 | 426.7 | 22.8 ± 2.1 | 388 | 30 ± 1.3 | 2.5 | 20 ± 1.2 | 1638.8 |
| COX-2 | 29.5 ± 0.2 | 29.8 ± 0.3 | 1 | 22.1 ± 0.5 | 146.5 | 22.6 ± 2.7 | 322 | 21.6 ± 0.6 | 238.7 | 28 ± 0.1 | 2.9 | 20.4 ± 0.4 | 384.5 |
| CXCL1 | 28.5 ± 0.6 | 28.5 ± 0.4 | 1.1 | 20.5 ± 0.5 | 208.9 | 19.9 ± 0.3 | 320.3 | 20.4 ± 0.5 | 239.2 | 25.6 ± 0.3 | 7 | 19.6 ± 0.6 | 297 |
| CCL1 | 33.3 ± 0.9 | 33.1 ± 0.8 | 1.4 | 27.6 ± 2.4 | 68.4 | 27 ± 0.8 | 270.2 | 26 ± 2.2 | 175.4 | 31.4 ± 1.5 | 4.2 | 26.1 ± 1.3 | 107.6 |
| IL-12p35 | 34.3 ± 1.2 | 34.2 ± 0.8 | 1.5 | 27.8 ± 0.9 | 86 | 28 ± 1.7 | 216.5 | 27.4 ± 0.9 | 129.5 | 32.7 ± 1.3 | 4.5 | 27 ± 0.6 | 116.5 |
| IL-1β | 25.1 ± 0.9 | 25.2 ± 0.6 | 1.1 | 17.7 ± 0.6 | 139 | 18.7 ± 2.3 | 211.3 | 17.5 ± 0.6 | 171.6 | 23 ± 0.75 | 5 | 16.9 ± 0.5 | 185 |
| IL-23p19 | 30 ± 1.1 | 30.3 ± 0.9 | 1 | 24 ± 1.2 | 53 | 22.2 ± 0.9 | 207.4 | 23.2 ± 1 | 97.2 | 28.5 ± 0.7 | 2.9 | 22.4 ± 1 | 134.8 |
| GM-CSF | 33 ± 0.7 | 32.6 ± 0.9 | 2 | 27.6 ± 0.8 | 40.6 | 27.8 ± 2 | 111.2 | 26.5 ± 0.7 | 88.2 | 29.5 ± 0.6 | 13.4 | 29 ± 0.5 | 11.8 |
| IL-10 | 34.5 ± 1 | 40 ± 0.0 | 0 | 31.2 ± 0.5 | 16 | 30.4 ± 2.8 | 91 | 29.7 ± 0.7 | 49 | 34.9 ± 0.3 | 2 | 29.5 ± 0.1 | 42 |
| CXCL10 | 28 ± 1.7 | 28 ± 1.5 | 1.5 | 25.8 ± 1.4 | 3.9 | 25.5 ± 4.3 | 47 | 25.5 ± 1.4 | 5 | 27.1 ± 1 | 2 | 18.25 ± 0.7 | 644.3 |
| NOS2 | 30.2 ± 0.2 | 30.6 ± 0.2 | 0.0 | 26.7 ± 0.5 | 9.5 | 26.7 ± 2.5 | 29.4 | 25.8 ± 0.6 | 18.8 | 33.5 ± 1.6 | 3.3 | 23.3 ± 0.4 | 77.3 |
| TNF-α | 23 ± 0.5 | 22.9 ± 0.2 | 1.2 | 18.8 ± 0.45 | 15.3 | 19.7 ± 2.5 | 25.36 | 18.5 ± 0.4 | 19.6 | 21.9 ± 0.3 | 2.2 | 17.3 ± 0.2 | 32.3 |
| CCL3 | 19 ± 0.4 | 18.9 ± 1.2 | 1.5 | 16 ± 0.4 | 6.8 | 17.4 ± 2.4 | 7.8 | 16.1 ± 0.7 | 6.9 | 18.2 ± 0.5 | 1.8 | 16.4 ± 0.5 | 3.8 |
| VCAM-1 | 29.6 ± 1.4 | 29.6 ± 1 | 1.2 | 27.3 ± 1 | 3.8 | 28 ± 2 | 6.5 | 26.9 ± 1 | 5.3 | 29 ± 1 | 1.6 | 25.4 ± 0.5 | 9.8 |
| ICAM-1 | 23.6 ± 0.7 | 23.5 ± 0.2 | 1.2 | 21.6 ± 0.5 | 3 | 23 ± 2.5 | 3.9 | 21.4 ± 0.6 | 3.9 | 23 ± 0.3 | 1.6 | 21.4 ± 0.4 | 3 |
BMDCs from WT mice were stimulated or not with IL-36Ra, IL-36α, IL-36β, IL-36γ, IL-1β, or LPS at a concentration of 100 ng/mL. Two hours after stimulation, mRNA was extracted and analyzed for mRNA expression using TaqMan low density array. The threshold cycle values (Ct) of each transcript represent the mean ± SD of triplicates in the same experiment. Hypoxanthine guanine phosphoribosyl transferase (HPRT) gene expression was assessed as an endogenous reference and used for fold increase normalization. The stimulatory activities of IL-36R ligands, IL-1β, or LPS were measured in fold increase compared with the nonstimulated cells. Data show only cytokines, chemokines, or growth factors significantly induced (P < .05) by the different stimuli compared with unstimulated cells. Results are representative of 3 independent experiments with similar results.
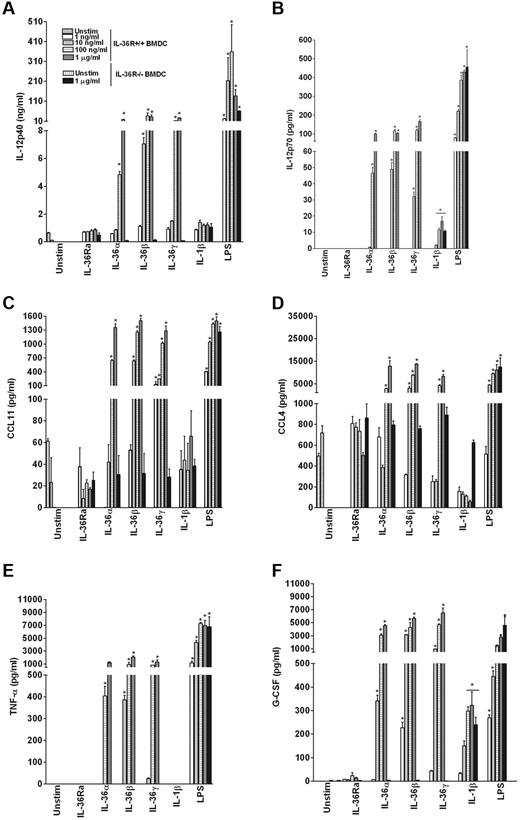
The effect of IL-36 on the production of cytokines was also determined at the protein level by multiplex assays in the 3-day culture supernatants. As shown in Figure 3, IL-36α, IL-36β, and IL-36γ dose-dependently stimulated production of IL-12, CCL11, CCL4, TNF-α, and G-CSF. Additional cytokines and chemokines, including IL-6, CXCL1, IL-9, IL-1α, IL-13, IL-1β, and IL-10, were also significantly induced by IL-36α, IL-36β, and IL-36γ (supplemental Table 2). Proteins for other highly induced mRNAs, such as COX-2, CCL1, CXCL10, and IL-23, were not measured or were below the sensitivity of the multiplex assays (IL-23). Cytokine production was not induced in IL-36R−/− BMDCs stimulated with 1 μg/mL of IL-36α, IL-36β, or IL-36γ, whereas IL-36R−/− cells exhibited similar responses as WT cells to LPS (Figure 3).
IL-36α, IL-36β, and IL-36γ specifically up-regulate the production of proinflammatory cytokines in BMDCs. BMDCs from WT or IL-36R−/− mice were incubated in the absence or presence of the indicated concentrations of IL-36Ra, IL-36α, IL-36β, IL-36γ, IL-1β, or LPS. Supernatants were collected after 72 hours of stimulation for the determination of cytokine levels: IL-12 p40 (A), IL-12 p70 (B) CCL11 (C), CCL4 (D), TNF-α (E) and G-CSF (F) by multiplex analysis. Data are shown from one of 2 independent experiments with similar results. Error bars represent SD of triplicates in the same experiment. *P < .05 (Student t test), IL-36, IL-1β, or LPS stimulation significantly differ from unstimulated cells.
IL-36α, IL-36β, and IL-36γ specifically up-regulate the production of proinflammatory cytokines in BMDCs. BMDCs from WT or IL-36R−/− mice were incubated in the absence or presence of the indicated concentrations of IL-36Ra, IL-36α, IL-36β, IL-36γ, IL-1β, or LPS. Supernatants were collected after 72 hours of stimulation for the determination of cytokine levels: IL-12 p40 (A), IL-12 p70 (B) CCL11 (C), CCL4 (D), TNF-α (E) and G-CSF (F) by multiplex analysis. Data are shown from one of 2 independent experiments with similar results. Error bars represent SD of triplicates in the same experiment. *P < .05 (Student t test), IL-36, IL-1β, or LPS stimulation significantly differ from unstimulated cells.
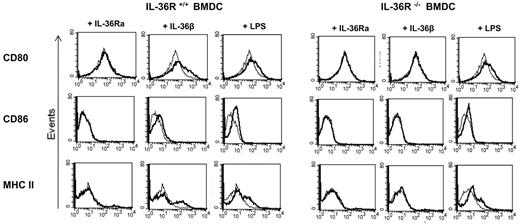
To investigate the effects of IL-36 on the capacity of DCs to present antigen and activate T cells, we measured cell surface expression of MHC class II, CD80, and CD86 by flow cytometry in WT and IL-36R−/− BMDCs cultured with IL-36Ra, IL-36β, or LPS at 100 ng/mL for 24 hours. Unstimulated CD11c+ BMDCs exhibited high CD80, but not CD86 and MHC class II surface expression. Stimulation with IL-36β up-regulated CD80, CD86, and MHC class II expression in WT BMDCs (Figure 4 left panels) but not in IL-36R−/− cells (Figure 4 right panels), whereas both WT and IL-36R−/− responded to LPS. Similar data were observed after IL-36α and IL-36γ stimulation (data not shown). Moreover, IL-36α, IL-36β, and IL-36γ were able to up-regulate CD40 expression in WT BMDCs but not in IL-36R−/− cells (data not shown). By contrast, we did not observe any effect of IL-36Ra. Thus, IL-36α, IL-36β, and IL-36γ trigger DC maturation resulting in increased cell surface MHC class II, CD80, and CD86 expression.
Up-regulation of costimulatory molecules by IL-36β and LPS in IL-36R +/+ BMDCs. BMDCs from WT (left panels) and IL-36R−/− (right panels) mice were either not stimulated (thin gray line) or stimulated with 100 ng/mL of IL-36Ra, IL-36β, or LPS (thick black line) for 24 hours. Cells were then stained for CD80, CD86, or MHC II (I-Ab) before analysis by flow cytometry. Histograms were gated on CD11c+ cells. Data are representative of 1 experiment of 2.
Up-regulation of costimulatory molecules by IL-36β and LPS in IL-36R +/+ BMDCs. BMDCs from WT (left panels) and IL-36R−/− (right panels) mice were either not stimulated (thin gray line) or stimulated with 100 ng/mL of IL-36Ra, IL-36β, or LPS (thick black line) for 24 hours. Cells were then stained for CD80, CD86, or MHC II (I-Ab) before analysis by flow cytometry. Histograms were gated on CD11c+ cells. Data are representative of 1 experiment of 2.
Enhanced cytokine production by IL-36 in activated CD4+ T cells
As described in “Specific stimulatory effect of IL-36 in BMDCs,” CD4+ T cells also express IL-36R mRNA. To further investigate whether IL-36R is differentially expressed in CD4+ T-cell subsets, total mRNA was extracted from splenic CD4+ T cells and in vitro differentiated Th1, Th2, and Th17 cells and analyzed by quantitative RT-PCR analysis. As shown in Figure 5A, total CD4+ T cells from the spleen, as well as Th1 and Th2 cells, express IL-36R mRNA, whereas Th17 cells express very low levels of IL-36R mRNA. In contrast, the IL-1 family receptors IL-1R1, IL-18Rα, and ST2 were predominantly expressed in Th17, Th1, and Th2 cells, respectively (data not shown). To investigate whether IL-36 could stimulate CD4+ T cells in vitro, purified CD4+ T cells were activated with anti-CD3/anti-CD28 antibodies and cultured for 3 days in absence or presence of increasing concentrations (1 ng/mL to 1 μg/mL) of the truncated IL-36 or IL-1β. Strikingly, and consistent with the pattern of IL-36R expression, addition of IL-36 potently induced production of IFN-γ (Figure 5B) and IL-4 (Figure 5C) by purified CD4+ T cells activated with anti-CD3/anti-CD28 antibodies, whereas it induced less IL-17 production compared with IL-1β (Figure 5D). The specificity of the stimulatory effect of IL-36 was confirmed using IL36R−/− CD4+ T cells cultured in the presence of 1 μg/mL of IL-36α, IL-36β, IL-36γ, or IL-1β (Figure 5B-D black bars). Of note, as shown in supplemental Figure 3, IL-36Ra significantly inhibited the effect of IL-36α, IL-36β, or IL-36γ on IFN-γ production when added at the concentration of 1 μg/mL but was devoid of inhibitory effect on IL-1β activity (supplemental Figure 3).
Expression of IL-36R in CD4+ T-cell subsets and effect of IL-36 on cytokine production by cultured T cells. (A) Quantification of IL-36R mRNA by quantitative RT-PCR in CD4+ T cells and in CD4+ T-cell subsets. Total mRNA was isolated from total splenic CD4+ T cells, Th1 cells, Th2 cells, and Th17 cells for quantitative RT-PCR analysis. Results represent IL-36R mRNA levels normalized for GAPDH expression. Error bars represent the SD of the mean of 3 independent experiments. (B-D) Dose-dependent effect of IL-36 in CD4+ T cells isolated from WT and IL36R−/− mice. Total splenic CD4+ T cells (1 × 105 cells/well) were seeded in 96-well plates precoated with anti-CD3/anti-CD28 mAb (0.5 μg/mL) and cultured in the absence (Unstimulated) or presence of the indicated concentrations of IL-36Ra, IL-36α, IL-36β, and IL-36γ for 72 hours. IFN-γ (B), IL-4 (C), and IL-17 (D) levels were measured in culture supernatants by ELISA. Error bars represent the SD of the means of triplicates in the same experiment. *P < .05 (Student t test), IL-36 or IL-1β stimulation significantly differs from unstimulated cells.
Expression of IL-36R in CD4+ T-cell subsets and effect of IL-36 on cytokine production by cultured T cells. (A) Quantification of IL-36R mRNA by quantitative RT-PCR in CD4+ T cells and in CD4+ T-cell subsets. Total mRNA was isolated from total splenic CD4+ T cells, Th1 cells, Th2 cells, and Th17 cells for quantitative RT-PCR analysis. Results represent IL-36R mRNA levels normalized for GAPDH expression. Error bars represent the SD of the mean of 3 independent experiments. (B-D) Dose-dependent effect of IL-36 in CD4+ T cells isolated from WT and IL36R−/− mice. Total splenic CD4+ T cells (1 × 105 cells/well) were seeded in 96-well plates precoated with anti-CD3/anti-CD28 mAb (0.5 μg/mL) and cultured in the absence (Unstimulated) or presence of the indicated concentrations of IL-36Ra, IL-36α, IL-36β, and IL-36γ for 72 hours. IFN-γ (B), IL-4 (C), and IL-17 (D) levels were measured in culture supernatants by ELISA. Error bars represent the SD of the means of triplicates in the same experiment. *P < .05 (Student t test), IL-36 or IL-1β stimulation significantly differs from unstimulated cells.
IL-36α, IL-36β, and IL-36γ enhance the effect of anti-CD3 on T-cell proliferation and cytokine production in cultured splenocytes
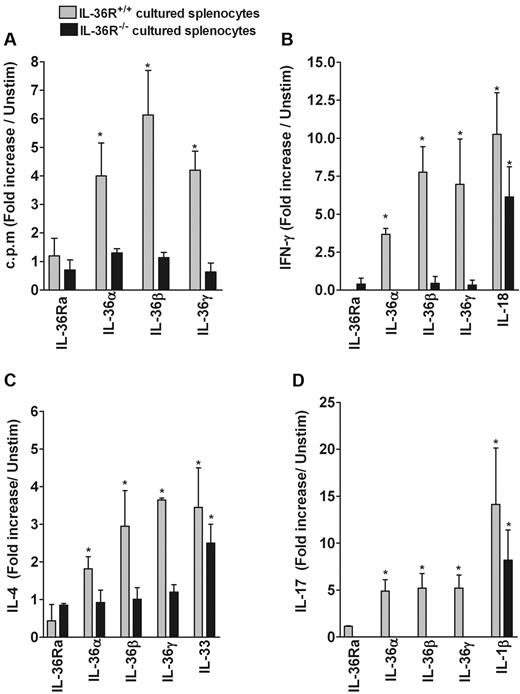
To assess whether IL-36α, IL-36β, and IL-36γ modulate splenocyte proliferation and immune response, splenocytes from WT and IL-36R−/− mice were cultured for 3 days in the presence of anti-CD3 mAb with or without 100 ng/mL of IL-36Ra, IL-36α, IL-36β, or IL-36γ. As shown in Figure 6A, IL-36 significantly enhanced the effect of anti-CD3 on T-cell proliferation in WT splenocytes (gray bars) but not in IL-36R−/− cells (black bars). In addition, production of IFN-γ by anti-CD3 activated splenocytes was markedly enhanced by IL-36α, IL-36β, and IL-36γ, to an extent comparable with that induced by IL-18 (Figure 6B). IL-36 also specifically and significantly stimulated production of IL-4 to an extent comparable with that of IL-33 (Figure 6C), whereas the induction of IL-17 was lower compared with IL-1β (Figure 6D). Of note, IL-36R−/− splenocytes did not produce significant levels of IFN-γ, IL-17, or IL-4 in response to IL-36. Moreover, several other cytokines, including IL-5, IL-6, IL-13, IL-3, and IL-10, were markedly induced in cultured splenocytes by IL-36R agonists, in particular by IL-36β and IL-36γ (from 60 to > 100-fold) compared with unstimulated cells (supplemental Table 3). In addition, several growth factors and chemokines, such as G-CSF, CXCL2, GM-CSF, vascular endothelial growth factor, leukemia inhibitor factor, and M-CSF were significantly enhanced with an induction ranging from 10- to 40-fold compared with unstimulated cells. Interestingly, IL-36Ra reduced the spontaneous production of cytokines by unstimulated cells. This result is consistent with the constitutive expression of IL-36β and IL-36γ mRNA by splenocytes (supplemental Figure 1C), whereas IL-36Ra and IL-36α mRNA was not detected (data not shown). Of note, cultured splenocytes did not respond to the different stimulatory effects of IL-36 in the absence of anti-CD3 mAb (data not shown). The specificity of the stimulatory effect of IL-36 was also confirmed using IL-36R−/− splenocytes (Figure 6; and data not shown).
Effect of IL-36 and other IL-1 family members on cell proliferation and cytokine production by cultured splenocytes. Splenocytes (2 × 105 cells/well) isolated from WT (gray bars) and IL-36R−/− mice (black bars) were cultured in 96-well-plates precoated with anti-CD3 mAb (0.08 μg/mL) and incubated in the absence or presence of 100 ng/mL IL-36Ra, IL-36α, IL-36β, IL-36γ, IL-1β, IL-33, or IL-18 for 72 hours. Results are shown as fold increase compared with unstimulated cells. (A) Proliferative responses were assessed by thymidine incorporation. IFN-γ (B), IL-4 (C), and IL-17 (D) production in culture supernatants was determined by ELISA. Error bars represent the SD of the means of triplicates in 3 independent experiments. *P < .05 (Student t test), IL-36, IL-18, IL-33, or IL-1β stimulation significantly differs from unstimulated cells.
Effect of IL-36 and other IL-1 family members on cell proliferation and cytokine production by cultured splenocytes. Splenocytes (2 × 105 cells/well) isolated from WT (gray bars) and IL-36R−/− mice (black bars) were cultured in 96-well-plates precoated with anti-CD3 mAb (0.08 μg/mL) and incubated in the absence or presence of 100 ng/mL IL-36Ra, IL-36α, IL-36β, IL-36γ, IL-1β, IL-33, or IL-18 for 72 hours. Results are shown as fold increase compared with unstimulated cells. (A) Proliferative responses were assessed by thymidine incorporation. IFN-γ (B), IL-4 (C), and IL-17 (D) production in culture supernatants was determined by ELISA. Error bars represent the SD of the means of triplicates in 3 independent experiments. *P < .05 (Student t test), IL-36, IL-18, IL-33, or IL-1β stimulation significantly differs from unstimulated cells.
IL-36β acts as an adjuvant to stimulate Th1 responses in vivo
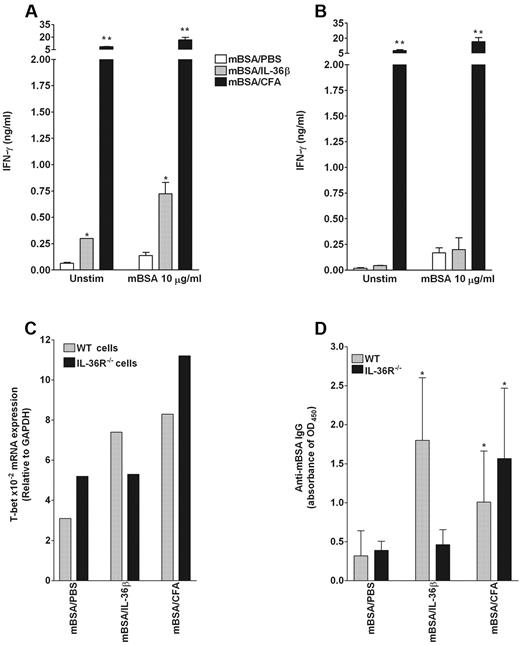
To examine the potential of IL-36β to act as an adjuvant in vivo, lymph node cells from WT and IL36R−/− mice, immunized intradermally with mBSA alone in PBS (negative control), mBSA in CFA (positive control) or mBSA plus IL-36β, were restimulated ex vivo with the specific antigen (mBSA). The levels of Th1 (IFN-γ), Th2 (IL-4), and Th17 (IL-17) cytokines in culture supernatants were determined by ELISA. As shown in Figure 7A, mBSA-stimulated cells from WT mice immunized with mBSA/IL-36β spontaneously produced significant levels of IFN-γ that were further enhanced in the presence of mBSA compared with mice injected with mBSA/PBS. The specificity of the IL-36β stimulatory effect was confirmed using mBSA-stimulated cells from IL-36R−/− mice immunized with mBSA/IL-36β (Figure 7B). In addition, lymph node cells from mBSA/CFA-immunized WT and IL-36R−/− mice spontaneously produced large amounts of IFN-γ that were further enhanced in the presence of mBSA (Figure 7A-B). Of note, lymph node cells from mice immunized with mBSA/IL-36β did not show a significant production of IL-17 and IL-4 after ex vivo stimulation with mBSA (data not shown).
IL-36β acts as an adjuvant to stimulate Th1 responses in vivo. (A-B) Specific IFN-γ production in lymph node cells from WT mice immunized with mBSA/IL-36β. WT (A) and IL-36R−/− (B) mice were immunized intradermally with mBSA plus PBS, mBSA plus IL-36β, or mBSA plus CFA. At day 21, draining lymph nodes from each group (n = 4) were collected, pooled, and cultured in the absence or presence of 10 μg/mL of mBSA. Culture supernatants were harvested after 3 days of incubation and then assayed for IFN-γ production in response to mBSA (A-B) by ELISA. Values are the mean ± SD of culture triplicates. *P < .05, compared with the value of mBSA/PBS-treated mice. **P < .005, compared with the value of mBSA/PBS-treated mice. (C) Quantification of T-bet mRNA expression by quantitative RT-PCR in draining lymph node cells. Total mRNA was extracted from pooled lymph node cells of each group (n = 4) for T-bet mRNA expression analysis by quantitative RT-PCR. Results represent T-bet mRNA levels relative to GAPDH. (D) Serum levels of anti-mBSA IgG in WT (gray bars) and IL-36R−/− (black bars) immunized mice. The levels of anti-mBSA IgG were determined by ELISA. Results are expressed as mean ± SD, optical density (OD) units from each group. *P < .05, compared with the value of mBSA/PBS-treated mice.
IL-36β acts as an adjuvant to stimulate Th1 responses in vivo. (A-B) Specific IFN-γ production in lymph node cells from WT mice immunized with mBSA/IL-36β. WT (A) and IL-36R−/− (B) mice were immunized intradermally with mBSA plus PBS, mBSA plus IL-36β, or mBSA plus CFA. At day 21, draining lymph nodes from each group (n = 4) were collected, pooled, and cultured in the absence or presence of 10 μg/mL of mBSA. Culture supernatants were harvested after 3 days of incubation and then assayed for IFN-γ production in response to mBSA (A-B) by ELISA. Values are the mean ± SD of culture triplicates. *P < .05, compared with the value of mBSA/PBS-treated mice. **P < .005, compared with the value of mBSA/PBS-treated mice. (C) Quantification of T-bet mRNA expression by quantitative RT-PCR in draining lymph node cells. Total mRNA was extracted from pooled lymph node cells of each group (n = 4) for T-bet mRNA expression analysis by quantitative RT-PCR. Results represent T-bet mRNA levels relative to GAPDH. (D) Serum levels of anti-mBSA IgG in WT (gray bars) and IL-36R−/− (black bars) immunized mice. The levels of anti-mBSA IgG were determined by ELISA. Results are expressed as mean ± SD, optical density (OD) units from each group. *P < .05, compared with the value of mBSA/PBS-treated mice.
To confirm that IL-36β promotes Th1 cell polarization in vivo, the levels of T-bet mRNA were determined in lymph node cells from WT and IL-36R−/− mice immunized with mBSA/PBS, mBSA/IL-36β, and mBSA/CFA. As illustrated in Figure 7C, T-bet mRNA expression was enhanced in lymph node cells from WT mice immunized with mBSA/IL-36β compared with those injected with mBSA/PBS (gray bars). By contrast, there was no difference in T-bet mRNA levels in IL-36R−/− mice immunized with mBSA/IL-36β and mBSA/PBS (Figure 7C black bars). In contrast, T-bet mRNA levels were increased in both WT and IL-36R−/− mice immunized with mBSA/CFA. We did not observe any specific increase of GATA-3 and RORγt mRNA levels in lymph node cells from WT mice immunized with mBSA/IL-36β (data not shown). These results show that IL-36β specifically elicits Th1-type cytokine responses in vivo. Finally, anti-mBSA IgG levels were determined in the serum of WT and IL-36R−/− mice immunized with mBSA/PBS, mBSA/IL-36β, and mBSA/CFA. As shown in Figure 7D, mBSA-specific IgG levels were significantly induced in WT mice immunized with mBSA/IL-36β compared with those injected with mBSA/PBS (gray bars), but not in IL-36R−/− mice immunized with mBSA/IL-36β (black bars).
Discussion
The IL-1 superfamily expanded 10 years ago by the discovery of 7 new members.3,11 Three of these ligands (IL-36α, IL-36β, and IL-36γ) were shown to activate signaling pathways similar to those induced by IL-1 in vitro in an IL-36R- and IL-1RAcP–dependent manner.12,13 However, little is known regarding the expression pattern and the biologic function of IL-36R ligands. The significant new findings of this study are that IL-36R is expressed constitutively in primary immune cells, such as DCs, CD4+ T lymphocytes, and macrophages, but not in B cells and CD8+ T cells. Both BMDCs and CD4+ T cells are able to significantly and specifically respond to IL-36α, IL-36β, and IL-36γ, enhancing Th responses in activated CD4+ T cells and splenocytes in vitro. Moreover, we show that IL-36β acts as an adjuvant to stimulate Th1 responses in vivo. Finally, IL-36Ra specifically inhibits the stimulatory activities of IL-36R agonists in a dose-dependent manner similar to that previously described for IL-1Ra.
In our search for cells responding to IL-36, we observed high IL-36R expression in keratinocytes, in agreement also with the presence of IL-36 in the epithelial barriers of the body.6,12,13 This finding suggests that IL-36 may exert similar functions as IL-1α or IL-33 to promote early inflammatory responses to tissue injury or infection.23-26 Of note, excessive IL-36α expression in the skin leads to keratinocyte proliferation and skin inflammation independent of the presence of functional T or B cells, thus indicating a direct stimulation of autocrine proinflammatory responses.18 According to our results, DCs appear to be another major cell target of IL-36. Indeed, IL-36α, IL-36β, and IL-36γ specifically enhanced DC maturation by inducing the expression of MHCII and the costimulation molecules CD80/CD86. Moreover, IL-36 also stimulated the production of various cytokines, chemokines, adhesion molecules, and proinflammatory mediators. It is possible that IL-36R agonist ligands are involved in the activation of innate and adaptive immune responses. DCs play a critical role in the balance between tolerance and immunity, bridging the innate and adaptive branches of the immune system.27 Most importantly, depending on the nature of microbial stimuli or endogenous innate signals, DCs also have the ability to induce different classes of Th cells.28 We observed that, in response to IL-36, BMDCs produce both IL-12p40 and IL-12p35 subunits of IL-12, which drives the polarization of naive precursors into Th1 cells. We also observed a specific induction of IL-1β, IL-6, TNF-α, and IL-23p19, cytokines that have been shown to be involved in the generation of Th17 cells.29-32 In human monocyte-differentiated DCs, IL-36 induces IL-12p40, IL-23p19, IL-1α, IL-1β, andIL-6 but induces only a very small amount of IL-12p35 (data not shown). These differences between mouse and human models might reflect biologic differences between the 2 species as well as differences in the differentiation protocol or origin of the cells (eg, bone marrow cells for mouse DCs vs peripheral blood monocyte for human DCs) and remain to be further investigated.
In the present study, we have also examined the regulation of IL-36 expression. Our data demonstrated that IL-36γ mRNA is constitutively present in BMDCs, whereas the expression of IL-36α mRNA is inducible in these cells, suggesting the existence of an amplification loop. Blumberg et al previously described such a positive feedback in mouse skin with cytokines, such as IL-17, IL-22, IL-23, and TNF, inducing IL-36α and IL-36γ, which, in turn, amplify proinflammatory cytokines.33 In addition, it has been reported that TNF-α, IL17A, and IL-1α dose-dependently induced IL-36Ra, IL-36β, and IL-36γ mRNA expression in normal human keratinocytes.25
BMDCs were highly responsive to IL-36, whereas other IL-1 family members exhibited only weak or no stimulatory effects. With the exception of IL-18, all the members of the IL-1 family of cytokines use IL-1RAcP as common coreceptor and stimulate similar intracellular signals.2 The responsiveness of different cell types to IL-1 members thus mainly depends on the relative expression of their specific receptors. For instance, we have previously shown that IL-1β exerts more potent stimulatory effects than IL-36β on human synovial fibroblast and human articular chondrocytes,17 whereas IL-33 was devoid of any effects on human synovial fibroblasts.34 Conversely, bone marrow-derived mast cells responded to IL-33,35 but not to IL-1 or IL-36β (D.T.-A., C.G. and G.P., unpublished results, October 2007).
Among CD4+ T cells, Th1 and Th2 expressed relatively high levels of IL-36R mRNA, whereas Th17 cells had the lowest levels. IL-36α, IL-36β, and IL-36γ induced the production of IFN-γ, IL-4, and IL-17 by cultured CD4+ T cells and splenocytes; however, induction of IFN-γ and IL-4 was stronger than that of IL-17. This finding may be related to the lower expression of IL-36R in polarized Th17 compared with Th1 and Th2 cells. Moreover, it is possible that the effect of IL-36 alone on cultured CD4+ T cells and splenocytes is not sufficient to allow optimal IL-17 induction, which may require the presence of additional factors.
Quantitative RT-PCR analysis indicates that low levels of IL-36β mRNA were detected in CD4+ T cells, whereas all other IL-36R ligand mRNAs were absent (data not shown). In contrast with our finding, it has been reported that human T cells produce IL-36α but not IL-36β mRNA in the absence or presence of stimulation with anti-CD3/anti-CD28 antibodies.10 IL-36γ mRNA was readily detectable in cultured splenocytes with and without the presence of anti-CD3 and/or anti-CD28 antibodies. Consistent with this finding, the spontaneous production of several cytokines by splenocytes was markedly decreased by the addition of IL-36Ra, thus suggesting that IL-36Ra exerts inhibitory activities on endogenously produced IL-36γ. We have investigated the putative antagonist effects of IL-36Ra and observed that the stimulatory effects of IL-36α, IL-36β, and IL-36γ were antagonized specifically by IL-36Ra in BMDCs and CD4 T cells. This finding is in agreement with earlier findings showing that IL-36Ra inhibits IL-36γ–induced NF-κB activation in Jurkat T cells overexpressing IL-36R.13 However, others could not reproduce this inhibitory effect of IL-36Ra in other experimental systems. The biochemical and functional characterization of bioactive IL-36Ra has recently been described (Towne et al, manuscript submitted). Using bioactive recombinant IL-36Ra, we have confirmed that IL-36Ra should be used in 100- to 1000-fold molar excess to inhibit the effects of IL-36. This finding is consistent with previous data on the inhibitory effect of IL-1Ra on IL-1.22 Further studies will be needed to define in which pathologic conditions IL-36Ra administration will provide a useful therapeutic effect. Another attractive therapeutic application is the possibility of direct use of IL-36 as an adjuvant. Indeed, many cytokines, including IL-1 family members, have been previously used as vaccine adjuvants to enhance primary and memory immune responses against certain cancers and infectious diseases.2,36-38
In conclusion, our study shows that IL-36 exerts stimulatory effects on DC and CD4+ T cells leading to a predominant Th1 response in vitro and in vivo. Therefore, our findings indicate that these cytokines may represent potential targets for immune-mediated inflammatory conditions or, alternatively, could be used as adjuvants in vaccination.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Arun Kamath (Center of Vaccinology, University of Geneva School of Medicine) for helpful discussion on the development of the in vivo immunization protocol.
This work was supported by the Swiss National Foundation (grant 310030-135195, C.G.; and grant 310030-134691, G.P.), the Rheumasearch Foundation, and the Institute of Arthritis Research.
Authorship
Contribution: S.V. planned studies, performed experiments, analyzed data, and wrote the manuscript; G.P. analyzed data and wrote the manuscript; C.L., P.M., D.T.-A., E.R., F.R., and H.D. performed experiments and analyzed data; F.S. and J.E.S. analyzed data and wrote the manuscript; and C.G. supervised the project, planned studies, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The salary of S.V. was supported by the Novartis Foundation (postdoctoral fellowship grant). J.E.S. and H.D. are employees of Amgen and own Amgen stock and/or stock options. The remaining authors declare no competing financial interests.
Correspondence: Cem Gabay, Division of Rheumatology, University Hospitals of Geneva, 26 Avenue Beau-Séjour, 1211 Geneva 14, Switzerland; e-mail: cem.gabay@hcuge.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal