Abstract
Deficiencies in transmembrane activator and CAML interactor (TACI) result in common variable immune deficiency, a syndrome marked by recurrent infections with encapsulated microorganisms, impaired production of antibodies, and lymphoproliferation. How TACI promotes antibody production and inhibits lymphoproliferation is not understood. To answer this question, we studied the generation of immunity to protein antigens in both TACI-deficient and TACI-proficient mice. We show that TACI promotes sustained Blimp-1 expression by B cells responding to antigen, which in turn limits B-cell clonal expansion and facilitates differentiation of long-lived antibody-secreting cells. Short-term IgG secretion occurs independently of TACI as DNA double-strand breaks associated with isotype class switching induce Blimp-1 transiently, independently of TACI. Our results showing that TACI induces and maintains Blimp-1 provide, for the first time, a unified molecular and cellular mechanism explaining the primary features of common variable immune deficiency, exquisite vulnerability to infection with encapsulated organisms, lymphoproliferation, and hypogammaglobulinemia.
Introduction
In humans, mutations in the TNFRSF13B gene that encodes transmembrane activator and CAML interactor (TACI) is thought to be the cause of 7% to 21% of cases of common variable immune deficiency (CVID), the primary immunodeficiency most commonly encountered in clinical practice. CVID is a heterogeneous syndrome caused by antibody deficiency manifested in late childhood or early to mid-adulthood. CVID patients often present with recurrent infections of the respiratory tract resulting from encapsulated organisms, such as Streptococcus pneumoniae and Haemophilus influenzae or Mycoplasma.1 Paradoxically, some CVID patients develop autoimmune diseases, the most common of which are hemolytic anemia and autoimmune thrombocytopenic purpura. CVID patients are at a high risk to develop neoplasms, primarily lymphomas.2 How TACI deficiency results in antibody deficiencies on the one hand, and lymphoproliferation, autoimmunity, and malignancy on the other, is not understood.
Animal models of TACI deficiency have replicated some of the features of CVID associated with TNFRSF13B mutations in human subjects. Thus, von Bulow et al3 found that TACI-deficient animals had severely decreased antibody responses to polysaccharides and enlarged spleens because of the increased number of B cells, but they did not observe defective antibody response to protein antigens, autoimmune manifestations, or malignancies. Yan et al4 also found in independently generated TACI-deficient mice, expansion of the mature B cell compartment, including follicular, marginal zone, and transitional B cells, causing enlarged B-cell follicle areas and deficient antibody responses to T-independent type II antigens. In a follow-up study of aging TACI-deficient mice, Seshasayee et al5 reported lymphomas, lymphocytic infiltration of vital organs, and systemic lupus erythematosus with membranoproliferative glomerulonephritis. To reconcile opposite manifestations of TACI deficiency, lymphoproliferation, autoimmunity, and hypogammaglobulinemia, we6 proposed that TACI has dual functions, promoting B-cell differentiation on the one hand, and on the other limiting proliferation and thus decreasing the risk of autoimmunity and cancer. In this manuscript, we identify the molecular mechanisms responsible.
Whether or not TACI promotes antibody secretion by enhancing plasma cell differentiation has been the subject of controversy. We6 found that lack of TACI on B cells impaired antibody production in response to polysaccharides or lipopolysaccharide (LPS) resulting from a defect in differentiation of antibody-secreting cells (ASCs). Ozcan et al7 showed that “a proliferation inducing ligand” (APRIL) synergized with LPS in promoting plasma cell differentiation, and Castigli et al8 showed that APRIL stimulated immunoglobulin production induced by anti-CD40 and anti–IL-4 by engaging TACI, suggesting that TACI promotes antibody secretion in response to T cell–dependent signals. In contrast, Sakurai et al9 concluded that TACI inhibited Blimp-1 expression and IgG secretion by B cells activated by CD40L, suggesting that TACI inhibits responses to T cell–dependent stimuli. Previous work in TACI-deficient mice revealing normal serum IgG and normal antibody responses to vaccination with protein antigens3,4 challenged the idea that TACI promotes antibody production in response to protein antigens. Because the pronounced hypogammaglobulinemia observed in human subjects with mutations in the gene encoding TACI suggested that TACI might promote antibody production more broadly than earlier studies indicated,3,4 we undertook to examine the molecular mechanisms responsible for the phenotype of TACI deletion mutants. Results indicate that TACI on B cells induces sustained Blimp-1 expression, which in turn limits clonal expansion and enhances differentiation of ASCs. We found that TACI promotes the generation and/or maintenance of long-lived plasma cells. Thus, a single molecular event explains both activating and inhibiting functions of TACI and provides a logical basis for the manifestations of CVID resulting from defective TACI.
Methods
Mice and immunizations
C57BL/6 mice were purchased from The Jackson Laboratory. Quasi-monoclonal (QM)–, TACI-wt, and QM-TACI-knockout (ko) mice were previously described.6,10,11 Mice were housed in a specific pathogen-free facility at the University of Michigan. All animal procedures were approved by the University of Michigan Committee on Use and Care of Animals. Mice were immunized with 100 μg of (4-hydroxy-3-nitrophenyl) acetyl (NP) conjugated to ovalbumin (NP-OVA; Biosearch Technologies) in an emulsion prepared with incomplete Freund adjuvant (Difco Laboratories). Mice were bled before immunization and every 7 days after immunization for 8 weeks.
Adoptive transfer
TACI-proficient or TACI-deficient B cells or T cells were isolated from the spleens of mice, with isolation kits (Miltenyi Biotec). A total of 5 million B cells were mixed with 5 million T cells and injected in the tail vein of C57BL/6-TACI-ko mice. Sera and spleens were collected 14 days after immunization.
Cell lines and culture conditions
The 18.81 cells were cultured according to published methods.12 Recombinant mouse APRIL was purchased from PeproTech.
Irradiation of cells
B cells isolated from spleens of TACI-ko mice or 18.81 cells12 were irradiated in the Experimental Irradiation Core of the University of Michigan Comprehensive Cancer Center. Cells received 1 Gy or 4 Gy delivered at a dose rate of 480.8 cGy/min. Irradiated cells were cultured in supplemented RPMI 1640 medium with 5 g/mL LPS (Sigma-Aldrich; B cells) or without LPS (18.81 cells). Cells were collected to extract RNA 24 hours or 48 hours after irradiation.
DNA double-strand breaks were identified 1 hour after irradiation by enumerating γH2AX foci identified with a rabbit anti–mouse γH2AX antibody (Abcam) detected with FITC-conjugated goat anti–rabbit IgG antibody (Jackson ImmunoResearch Laboratories). Digital images were obtained with a Leica DMI6000 B microscope and LAS AF6000 Core Application Software (Leica Microsystems).
TACI-expression vectors
Mouse full-length TACI fragment was amplified from cDNA clone BC141867 and cloned in pIRES2-enhanced green fluorescent protein (EGFP) vector (Clontech) to originate a single bicistronic mRNA encoding TACI and EGFP. TACI expression on the cell surface was confirmed by flow cytometric analysis (data not shown).
ELISA
ELISPOT
ELISPOT was performed as described previously.6 The number of spots of NP-specific IgM- or IgG-secreting cells was counted by ImmunoSpot Professional Analyzer Version 5.0.9 (Cellular Technology) and confirmed by direct observation.
RT-PCR and quantitative PCR
Reverse transcription was prepared with SuperScript III First-Strand Synthesis System (Invitrogen). Quantitative PCR reactions were run on Mastercycler ep realplex (Eppendorf), quantified by FastStart Universal SYBR Green Master (Roche Diagnostics) incorporation, and results were expressed relative to β-actin. Primer sequences and amplification conditions are detailed in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Flow cytometry
FITC-conjugated anti–mouse CD69 (H1.2F3), PE-conjugated anti–mouse IgMa, (DS-1), and CD19 (1D3) antibodies were purchased from BD Biosciences. Anti-idiotypic antibodies were kindly supplied by Dr Imanishi-Kari (Tufts University, Boston, MA). Data were acquired with BD FACSCanto II (BD Biosciences) and analyzed using FACSDiva Version 6.1.1 software (BD Biosciences).
Immunohistochemistry
Peyer patches removed from mice were covered with OCT (Sakura), immediately frozen in liquid nitrogen, and stored at −80°C. Five-micron-thick sections were mounted on positively charged microscope slides (Fisher Scientific). Sections were air-dried for 30 minutes at room temperature, fixed with acetone for 10 minutes at −20°C, and air-dried for 10 minutes at room temperature. The specimens were pretreated with 0.3% hydrogen peroxide to quench the endogenous peroxidase activity, goat anti–rat IgG (Jackson ImmunoResearch Laboratories) followed by development using Vectastain Elite ABC Kit (Vector Laboratories). The specimens were counterstained with hematoxylin, dehydrated in graded ethanols, cleared in xylene. Digital images were obtained by Leica DM6000 B microscope (Leica) and with QCapture Pro Version 6.0.0 software (QImaging).
Results
TACI is necessary for the production and maintenance of serum immunoglobulin of IgM, IgG, and IgA isotypes
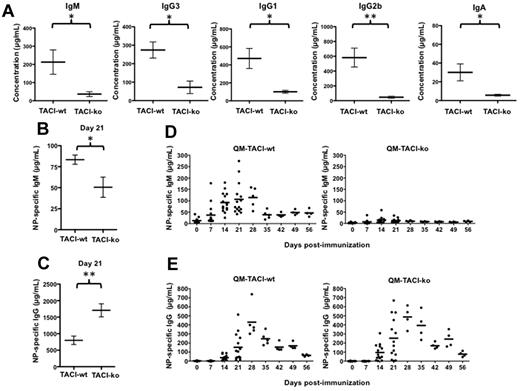
Deficiency of TACI in human subjects causes hypogammaglobulinemia. However, earlier studies in TACI-deficient mice3,4 failed to detect hypogammaglobulinemia, suggesting the possibility that murine and human TACI may have distinct functions. To verify earlier observations in mice, we quantified Ig isotypes in the serum of TACI-deficient and in wild-type mice, 8 to 12 weeks of age, housed in the same specific pathogen-free room at the University of Michigan. Our results (Figure 1A) show that, in contrast to earlier published results,3,4 the concentrations of all Ig isotypes were reduced in mice that lack TACI. Thus, C57BL/6-TACI-ko mice had concentrations of IgM in sera reduced by 12-fold, 5-fold less serum IgG1, 13-fold less IgG2b, 4-fold less IgG3, and 5-fold less IgA compared with wild-type mice. Because antibodies in the sera of mice maintained in specific pathogen-free facilities accumulate slowly from birth, earlier results may have failed to detect hypogammaglobulinemia3 resulting from immaturity of the immune system in the very young mice analyzed. Our results showing reduction in the concentrations of IgM, IgG, and IgA serum isotypes suggest that TACI may promote and/or help to maintain antibody responses to a variety of stimuli, including both polysaccharides and proteins.
TACI deficiency decreased serum IgM, IgG, and IgA and impaired antigen-specific IgM production in response to a protein antigen. (A) Sera IgM, IgG1, IgG3, IgG2b, and IgA quantified by ELISA. Sera were obtained from nonimmunized C57BL/6-TACI-wt (TACI-wt) or C57BL/6-TACI-ko (TACI-ko) mice. Concentrations were calculated by comparison with standard curves obtained with known concentrations of the respective isotypes. (B-C) Concentrations of NP-specific IgM (B) or IgG (C) determined by ELISA in the sera of C57BL/6-TACI-wt (TACI-wt) or C57BL/6-TACI-ko (TACI-ko) mice immunized with 100 μg NP-OVA 21 days earlier. TACI deficiency decreased NP-specific IgM but not NP-specific IgG. Concentrations were calculated by comparison with standard curves obtained with known concentrations of NP-specific monoclonal antibodies. (D-E) Concentrations of NP-specific IgM (D) or IgG1 (E) determined by ELISA in the sera of QM-TACI-wt or QM-TACI-ko mice after immunization with 100 μg NP-OVA. TACI deficiency decreased NP-specific IgM production and accelerated the production of NP-specific IgG. Concentrations were calculated by comparison with standard curves obtained with known concentrations of NP-specific monoclonal antibodies. Each dot represents results obtained from one mouse. Averages were compared by t test. Significant differences are noted: *P < .05; **P < .01.
TACI deficiency decreased serum IgM, IgG, and IgA and impaired antigen-specific IgM production in response to a protein antigen. (A) Sera IgM, IgG1, IgG3, IgG2b, and IgA quantified by ELISA. Sera were obtained from nonimmunized C57BL/6-TACI-wt (TACI-wt) or C57BL/6-TACI-ko (TACI-ko) mice. Concentrations were calculated by comparison with standard curves obtained with known concentrations of the respective isotypes. (B-C) Concentrations of NP-specific IgM (B) or IgG (C) determined by ELISA in the sera of C57BL/6-TACI-wt (TACI-wt) or C57BL/6-TACI-ko (TACI-ko) mice immunized with 100 μg NP-OVA 21 days earlier. TACI deficiency decreased NP-specific IgM but not NP-specific IgG. Concentrations were calculated by comparison with standard curves obtained with known concentrations of NP-specific monoclonal antibodies. (D-E) Concentrations of NP-specific IgM (D) or IgG1 (E) determined by ELISA in the sera of QM-TACI-wt or QM-TACI-ko mice after immunization with 100 μg NP-OVA. TACI deficiency decreased NP-specific IgM production and accelerated the production of NP-specific IgG. Concentrations were calculated by comparison with standard curves obtained with known concentrations of NP-specific monoclonal antibodies. Each dot represents results obtained from one mouse. Averages were compared by t test. Significant differences are noted: *P < .05; **P < .01.
To test whether TACI promotes antibody responses to protein antigens, we immunized TACI-ko or TACI-wt mice with a model protein-hapten conjugate NP-OVA. We immunized mice with NP-OVA mixed in incomplete Freund adjuvant rather than mixed with complete Freund adjuvant to avoid masking the protein-antigen specific primary responses by introducing bacterial antigens known to activate Toll-like receptors in polyclonal B cells independently of T-cell help. We found, as Figure 1B shows, that TACI deficiency severely blunts NP-specific IgM production in response to immunization. In contrast, TACI deficiency hastens the kinetics of NP-specific IgG production yielding 2.2-fold more IgG than wild-type mice 21 days after immunization (TACI-ko mice had 800 ± 127 μg/mL IgG and TACI-wt mice had 1708 ± 198 μg/mL IgG, P = .0051, Figure 1C). However, the increase in the concentration of IgG in sera of TACI-deficient mice was transient because by 35 days after immunization these animals had significantly less NP-specific IgG (830.6 μg/mL, on average), compared with wild-type mice (1329 μg/mL, on average). These results suggested that, in contrast to previously published reports,8 TACI deficiency does not impair early isotype class-switched antibody responses to proteins. Instead, TACI deficiency impairs sustained IgG responses after immunization.
To uncover the specific mechanism by which TACI promotes IgM antibody production and to avoid biases resulting from variability in the frequency of antigen-specific B cells, we took advantage of a mouse model engineered to increase the representation of a single B-cell clone in the B-cell repertoire. In QM mice, 80% of the peripheral B cells express an antibody that recognizes the hapten NP.10 In nonimmunized QM mice, there is very little NP-specific IgM (< 50 μg/mL) and NP-specific IgG is undetectable.10,14 This fact makes the QM mouse an ideal model to follow the development of NP-specific B cells in response to immunization. We immunized TACI-proficient QM mice (QM-TACI-wt) or TACI-deficient QM mice (QM-TACI-ko) with NP-OVA, as described. At the peak of the primary antibody response, 21 days after immunization, QM-TACI-ko mice had only 12% of the concentration of serum NP-specific IgM compared with wild-type mice (12.8 μg/mL, on average, in QM-TACI-ko mice and 106.6 μg/mL, on average, in wild-type; Figure 1D). As in mice with wild-type B cell repertoire, TACI deficiency in QM mice accelerated early NP-specific IgG production. By 21 days after immunization, QM-TACI-ko mice had 253.4 μg/mL NP-specific IgG1, whereas QM-TACI-wt mice had 152.7 μg/mL NP-specific IgG1 in sera (Figure 1E). Thus, TACI deficiency impairs the primary IgM response to protein antigens and enhances early IgG production.
TACI promotes differentiation of Ig-secreting cells in response to protein antigens
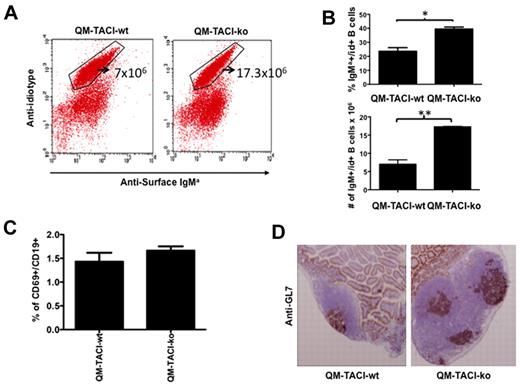
Inability to form IgM could be caused by decreased frequency of antigen-specific B cells, defective activation in response to immunization, or alternatively could reflect a selective defect in the differentiation of IgM B cells. To determine whether TACI deficiency decreased the proportion of antigen-specific B cells in the naive compartment, we detected NP-specific B cells in QM-TACI-wt and in QM-TACI-ko mice, 10 days after immunization. Figure 2A and B shows that QM-TACI-ko mice have 2.5-fold more NP-specific IgM positive B cells in the spleen than QM-TACI-wt mice, indicating that defective NP-specific IgM production is not the result of a decreased number of antigen-specific B cells in TACI-deficient mice. To test whether TACI deficiency impaired B-cell activation or clonal expansion in response to protein antigens, we measured the proportion of B cells expressing CD69 (an activation marker) and detected germinal center formation in Peyer patches after immunization. We found that the proportion of activated CD69+ B cells was comparable in QM-TACI-ko or in QM-TACI-wt mice (Figure 2C). We also observed that QM-TACI-ko mice had increased size and more germinal centers (GL7+, Ki67+) in the Peyer patches, compared with QM-TACI-wt mice (Figure 2D). These results suggested that TACI deficiency does not impair B-cell activation or clonal expansion after immunization with protein antigens.
TACI deficiency does not impair B-cell activation and germinal center formation. (A) The proportion of NP-specific B cells (idiotype-positive) in the spleen of QM-TACI-wt or QM-TACI-ko mice was analyzed by flow cytometry in mice immunized 10 days earlier. Splenocytes were stained with an anti–idiotypic antibody10 and with an anti–IgMa antibody that detects the allotype produced by the heavy chain-targeted allele. (B) Average proportions (above) or average number (below) of idiotype-positive cells obtained from 3 QM-TACI-wt or 3 QM-TACI-ko mice. Averages were compared by t test. Significant differences: *P < .05; **P < .01. (C) TACI deficiency does not decrease B-cell activation after immunization. Splenocytes were obtained from QM-TACI-wt or QM-TACI-ko mice immunized with 100 μg NP-OVA 10 days earlier. Splenocytes were stained with anti-CD19 and CD69 antibodies and analyzed by FACS. Activated B cells (CD19+, CD69+) were as frequent in TACI-proficient as in TACI-deficient mice. Average proportions of CD19+, CD69+ cells obtained from 3 QM-TACI-wt, or 3 QM-TACI-ko mice. Averages were compared by t test. Differences between groups were not significant. (D) TACI deficiency caused increases in size and number of germinal centers 10 days after immunization. Frozen sections of Peyer patches were stained with anti–GL7 antibody (brown), which specifically labels germinal center B cells. The figure is representative of 3 independent experiments. Digital images were obtained by Leica DM6000 B microscope (Leica) and with QCapture Pro Version 6.0.0 software (QImaging).
TACI deficiency does not impair B-cell activation and germinal center formation. (A) The proportion of NP-specific B cells (idiotype-positive) in the spleen of QM-TACI-wt or QM-TACI-ko mice was analyzed by flow cytometry in mice immunized 10 days earlier. Splenocytes were stained with an anti–idiotypic antibody10 and with an anti–IgMa antibody that detects the allotype produced by the heavy chain-targeted allele. (B) Average proportions (above) or average number (below) of idiotype-positive cells obtained from 3 QM-TACI-wt or 3 QM-TACI-ko mice. Averages were compared by t test. Significant differences: *P < .05; **P < .01. (C) TACI deficiency does not decrease B-cell activation after immunization. Splenocytes were obtained from QM-TACI-wt or QM-TACI-ko mice immunized with 100 μg NP-OVA 10 days earlier. Splenocytes were stained with anti-CD19 and CD69 antibodies and analyzed by FACS. Activated B cells (CD19+, CD69+) were as frequent in TACI-proficient as in TACI-deficient mice. Average proportions of CD19+, CD69+ cells obtained from 3 QM-TACI-wt, or 3 QM-TACI-ko mice. Averages were compared by t test. Differences between groups were not significant. (D) TACI deficiency caused increases in size and number of germinal centers 10 days after immunization. Frozen sections of Peyer patches were stained with anti–GL7 antibody (brown), which specifically labels germinal center B cells. The figure is representative of 3 independent experiments. Digital images were obtained by Leica DM6000 B microscope (Leica) and with QCapture Pro Version 6.0.0 software (QImaging).
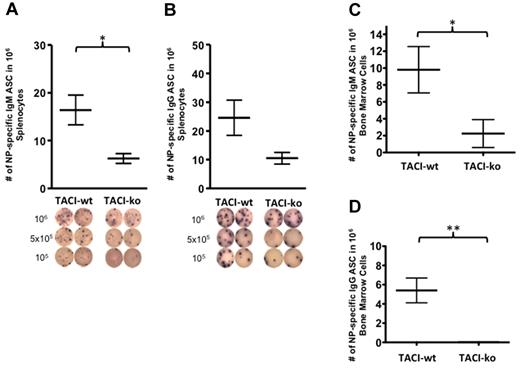
To test whether TACI promotes differentiation of NP-specific B cells into ASCs, we enumerated the NP-specific IgM or IgG ASCs in the spleen of C57BL/6-TACI-wt or C57BL/6-TACI-ko mice at the peak of the immune response, 21 days after immunization. TACI deficiency caused a significant decrease in the number of NP-specific IgM ASCs in the spleen (6.250 ± 1.03/106 B cells in ko mice and 16.40 ± 3.140/106 B cells in wt mice; Figure 3A). TACI deficiency also caused a reduction (albeit not statistically significant) in the number of NP-specific IgG ASCs in the spleen (10.50 ± 2.021/106 cells in ko and 24.60 ± 6.120/106 cells in wt mice; Figure 3B). To determine whether TACI deficiency impaired long-lived plasma cells, we enumerated antigen-specific ASCs in the bone marrow 56 days after immunization of QM-TACI-ko or QM-TACI-wt mice. QM-TACI-wt mice had on average 4-fold more antigen-specific IgM ASCs (Figure 3C) and at least 5-fold more IgG ASCs (Figure 3D) in the bone marrow than QM-TACI-ko mice. These results indicate that TACI is necessary for development or maintenance of long-lived ASCs.
TACI is required for differentiation of B cells into ASCs. TACI-ko animals had decreased NP-specific IgM ASCs or NP-specific IgG ASCs compared with wild-type. ASCs were enumerated in B cells isolated from the spleen (A-B) or in bone marrow cell suspensions (C-D) of C57BL/6-wt or C57BL/6-TACI-ko 21 days after immunization, by ELISPOT. Below the graphs, the diagrams show the ELISPOT wells from which the ASCs were enumerated and plated with 106, 5 × 105, or 105 cells, as indicated. Values were compared by t test. Significant differences: *P < .05; **P < .01. Data resulted from the analyses of 5 wild-type mice and 4 TACI-ko mice.
TACI is required for differentiation of B cells into ASCs. TACI-ko animals had decreased NP-specific IgM ASCs or NP-specific IgG ASCs compared with wild-type. ASCs were enumerated in B cells isolated from the spleen (A-B) or in bone marrow cell suspensions (C-D) of C57BL/6-wt or C57BL/6-TACI-ko 21 days after immunization, by ELISPOT. Below the graphs, the diagrams show the ELISPOT wells from which the ASCs were enumerated and plated with 106, 5 × 105, or 105 cells, as indicated. Values were compared by t test. Significant differences: *P < .05; **P < .01. Data resulted from the analyses of 5 wild-type mice and 4 TACI-ko mice.
TACI controls differentiation of Ig-secreting cells in a B cell-autonomous manner
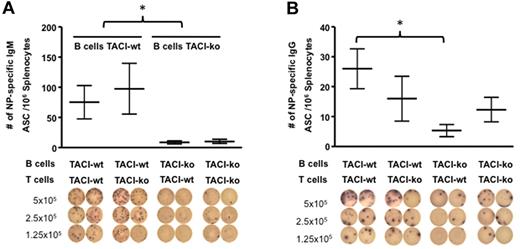
Antibody response to proteins requires T-cell help. Because, according to some studies, activated T cells express TACI,3 we wondered whether defective antibody secretion was the result of TACI deficiency on B cells, TACI deficiency on T cells, or both. To answer that question, we enumerated ASCs in C57BL/6-TACI-ko mice reconstituted with either QM-TACI-ko or QM-TACI-wt B cells, and C57BL/6-TACI-ko or C57BL/6-TACI-wt T cells. Chimeras were immunized with 100 μg of NP-OVA shortly after cell transfer. TACI-wt B cells were necessary and sufficient to produce NP-specific IgM and IgG secreting cells. Mice reconstituted with TACI-wt B and TACI-wt T cells had on average 75.3 NP-specific IgM ASCs/million B cells, whereas mice reconstituted with TACI-ko B cells and TACI-wt T cells had only 8.7 NP-specific IgM ASCs/million B cells in the spleen, 14 days after immunization (Figure 4A). Figure 4B shows that transfer of TACI-deficient B cells also resulted in a decreased number of NP-specific IgG ASCs. Thus, mice reconstituted with TACI-wt B and TACI-wt T cells had on average 26 NP-specific IgG ASC/million B cells, whereas mice reconstituted with TACI-ko B cells and TACI-wt T cells had only 5.3 NP-specific IgG ASCs/million B cells in the spleen, 14 days after immunization. Because in chimeras reconstituted with TACI-proficient B cells, the number of antigen-specific IgM or IgG ASCs in the spleen did not significantly differ in mice that had received TACI-wt (75.3 NP-specific IgM or 26 NP-specific IgG ASC/million B cells, respectively) or TACI-ko T cells (97.7 NP-specific IgM or 16 NP-specific IgG ASC/million B cells), we conclude that TACI expression by B cells determines Ig production autonomously (Figure 4A-B).
TACI deficiency decreases production of ASCs in a B cell–autonomous manner. (A-B) The number of ASCs was restored by adoptive transfer of TACI-wt B cells but not by transfer of TACI-wt T cells into TACI-deficient recipients. NP-specific IgM (A) or NP-specific IgG (B) ASCs were enumerated by ELISPOT of splenocytes 14 days after immunization, and after reconstitution by adoptive transfer of QM-TACI-wt or QM-TACI-ko B cells, and C57BL/6-wt or C57BL/6-TACI-ko T cells. Representative pictures of the ELISPOT wells are shown below each graph. Transfer efficiency was equivalent in all experiments and determined by enumerating IgMa-positive donor B cells by FACS analysis in the spleen of recipients at the time of analysis. The number of IgM-secreting cells was significantly decreased whenever reconstitution was done with TACI-ko B cells: *P = .016 (2-way ANOVA). The number of IgG-secreting cells in animals reconstituted with TACI-ko B cells and TACI-wt T cells was significantly decreased compared with animals reconstituted with TACI-wt B and T cells: *P = .041 (t test). Data resulted from analysis of 3 recipient mice for each type of transfer.
TACI deficiency decreases production of ASCs in a B cell–autonomous manner. (A-B) The number of ASCs was restored by adoptive transfer of TACI-wt B cells but not by transfer of TACI-wt T cells into TACI-deficient recipients. NP-specific IgM (A) or NP-specific IgG (B) ASCs were enumerated by ELISPOT of splenocytes 14 days after immunization, and after reconstitution by adoptive transfer of QM-TACI-wt or QM-TACI-ko B cells, and C57BL/6-wt or C57BL/6-TACI-ko T cells. Representative pictures of the ELISPOT wells are shown below each graph. Transfer efficiency was equivalent in all experiments and determined by enumerating IgMa-positive donor B cells by FACS analysis in the spleen of recipients at the time of analysis. The number of IgM-secreting cells was significantly decreased whenever reconstitution was done with TACI-ko B cells: *P = .016 (2-way ANOVA). The number of IgG-secreting cells in animals reconstituted with TACI-ko B cells and TACI-wt T cells was significantly decreased compared with animals reconstituted with TACI-wt B and T cells: *P = .041 (t test). Data resulted from analysis of 3 recipient mice for each type of transfer.
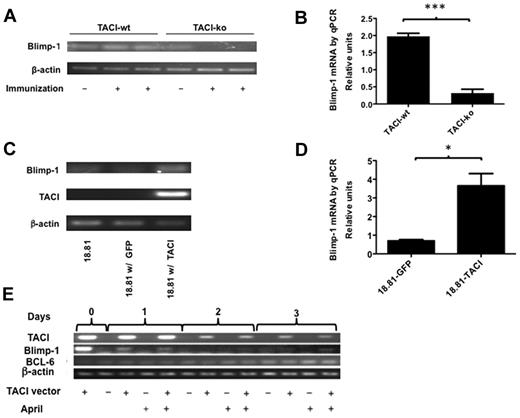
TACI induces Blimp-1 production by B cells
Differentiation of germinal center B cells requires cessation of B-cell proliferation and is at least in part dependent on Blimp-1 expression,15 even though initiation of B-cell differentiation may be Blimp-1 independent.16 Current thought supports a model in which Blimp-1 expression enhances gene-expression changes necessary for complete plasma cell maturation. Thus, Blimp-1, a transcriptional repressor, halts the cell cycle in part because of repressing PAX5, Myc, and BCL-6 and inducing X-box binding protein 1 (XBP1), which orchestrates the unfolded protein reaction needed to induce the secretory phenotype. We asked whether TACI may promote differentiation of IgM ASCs by increasing Blimp-1 expression in centrocytes. To test whether TACI deficiency impaired Blimp-1 expression and because Blimp-1 expression is controlled at the level of transcription, we first examined Blimp-1 mRNA in B cells obtained from spleens of QM-TACI-wt or QM-TACI-ko mice after immunization. Figure 5A and B shows that immunization increases Blimp-1 expression by 1.8-fold in TACI-proficient B cells, whereas it reduces expression of Blimp-1 by 0.6-fold in TACI-deficient B cells, relative to expression before immunization. The results suggested that TACI induces Blimp-1 on B-cell activation.
TACI is necessary and sufficient to induce Blimp-1 in B cells. (A-B) TACI deficiency abrogates Blimp-1 expression by B cells 14 days after immunization. Blimp-1 mRNA expression was detected by RT-PCR (A) and quantified by real-time PCR (B). Relative expression of Blimp-1 was calculated by determining the ratio of Blimp-1/β-actin in immunized B cells compared with the Blimp-1/β-actin ratio in B cells from nonimmunized mice. Results represent the mean ± SEM of 3 measurements/mouse of each of 4 mice/genotype. Values were compared by t test and found to be significantly different: ***P < .0001. (C-D) Expression of TACI is sufficient to induce Blimp-1. The 18.81 B cells were transfected with a vector encoding TACI and EGFP from a single bicistronic mRNA or with a control vector encoding only EGFP. RNA was isolated from sorted GFP-positive transfected cells, 24 hours after transfection and converted to cDNA. Blimp-1 mRNA expression was studied by RT-PCR, after 30 PCR cycles (C) or quantitative RT-PCR (D). Blimp-1 expression by 18.81 B cells expressing TACI was calculated relative to the Blimp-1 expression by 18.81 cells transfected with a GFP control vector. Results represent mean ± SEM of 3 separate measurements. Values were compared by t test and found to be significantly different: *P = .0103. (E) Blimp-1 and TACI expression decreased and BCL-6 expression increased with time in 18.81 B cells transfected with a TACI expression vector. The 18.81 B cells transfected with TACI and EGFP or with EGFP control vectors were cultured up to 3 days with or without 100 ng/mL APRIL, as indicated. TACI, BCL-6, and Blimp-1 mRNA expression was studied by RT-PCR, after 30 PCR cycles. Cells were collected at 0, 24, 48, and 72 hours in culture. Results are representative of 3 independent experiments.
TACI is necessary and sufficient to induce Blimp-1 in B cells. (A-B) TACI deficiency abrogates Blimp-1 expression by B cells 14 days after immunization. Blimp-1 mRNA expression was detected by RT-PCR (A) and quantified by real-time PCR (B). Relative expression of Blimp-1 was calculated by determining the ratio of Blimp-1/β-actin in immunized B cells compared with the Blimp-1/β-actin ratio in B cells from nonimmunized mice. Results represent the mean ± SEM of 3 measurements/mouse of each of 4 mice/genotype. Values were compared by t test and found to be significantly different: ***P < .0001. (C-D) Expression of TACI is sufficient to induce Blimp-1. The 18.81 B cells were transfected with a vector encoding TACI and EGFP from a single bicistronic mRNA or with a control vector encoding only EGFP. RNA was isolated from sorted GFP-positive transfected cells, 24 hours after transfection and converted to cDNA. Blimp-1 mRNA expression was studied by RT-PCR, after 30 PCR cycles (C) or quantitative RT-PCR (D). Blimp-1 expression by 18.81 B cells expressing TACI was calculated relative to the Blimp-1 expression by 18.81 cells transfected with a GFP control vector. Results represent mean ± SEM of 3 separate measurements. Values were compared by t test and found to be significantly different: *P = .0103. (E) Blimp-1 and TACI expression decreased and BCL-6 expression increased with time in 18.81 B cells transfected with a TACI expression vector. The 18.81 B cells transfected with TACI and EGFP or with EGFP control vectors were cultured up to 3 days with or without 100 ng/mL APRIL, as indicated. TACI, BCL-6, and Blimp-1 mRNA expression was studied by RT-PCR, after 30 PCR cycles. Cells were collected at 0, 24, 48, and 72 hours in culture. Results are representative of 3 independent experiments.
To determine whether expressing TACI was sufficient on its own to induce Blimp-1, we expressed TACI in Abelson murine leukemia virus-transformed (18.81) B cells12,17 and quantified Blimp-1 production in transfected cells. The choice of 18.81 B cells was motivated by the fact that the line exhibits some properties of germinal center B cells; 18.81 B cells proliferate very fast and undergo somatic hypermutation and Ig class switch recombination.12 We studied 18.81 cells transfected with vectors encoding TACI and GFP in a single bicistronic mRNA or with a control vector encoding GFP. To test whether TACI expression was sufficient to induce Blimp-1 expression, we measured Blimp-1 RNA in sorted TACI and/or GFP-expressing cells. Figure 5C and D shows that TACI expression induces Blimp-1 expression in 18.81 cells by > 3-fold, indicating that TACI induces Blimp-1 expression on its own. In addition, as TACI expression decreases over the course of 3 days, so does Blimp-1 expression, whereas BCL-6 expression increases (Figure 5E). These results suggest that TACI orchestrates the genetic changes necessary to initiate plasma cell differentiation.
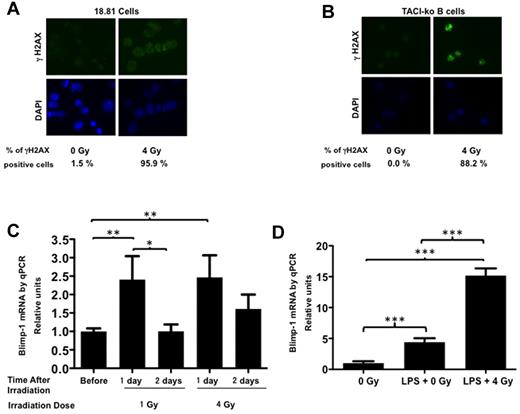
DNA double-strand breaks induce Blimp-1 production by TACI-deficient cells
TACI-deficient mice have less IgG in the sera compared with TACI-proficient mice, suggesting that TACI contributes to IgG production in the mouse, thus mimicking the hypogammaglobulinemia observed in human subjects with deficiencies in TACI. Surprisingly, measurements of antigen-specific IgG in TACI-deficient animals after immunization with a T-dependent antigen revealed hastened kinetics and at times increased concentration of IgG in the serum, compared with TACI-wt animals. Yet the number of antigen-specific ASCs in the spleen 3 weeks after immunization was reduced in TACI-deficient mice compared with TACI-proficient mice. Because class switch recombination requires double-strand breaks, we asked whether DNA double-strand breaks would induce Blimp-1 independently of TACI, thus promoting differentiation of IgG-secreting plasma cells. To test whether DNA double-strand breaks induce Blimp-1 expression, we irradiated 18.81 cells or TACI-ko B cells and detected γH2AX foci by immunofluorescence. Figure 6A and B shows that irradiation of 18.81 (A) or TACI-ko B cells (B) resulted in γH2AX foci, which are induced by DNA double-strand breaks.18 Next, we determined whether irradiation induced Blimp-1 expression by quantitative RT-PCR. Figure 6C shows that irradiation of 18.81 cells in the presence of TACI-Fc, which competes for the TACI ligands, induced Blimp-1 expression after 24 hours. Figure 6D shows that irradiation of TACI-deficient cells obtained from TACI-ko mice generated γH2AX foci and induced Blimp-1, demonstrating that DNA double-strand breaks induce Blimp-1 independently of TACI. Plasma cell differentiation requires sustained Blimp-1 expression, but induction of Blimp-1 by DNA double-strand breaks may be short lived (Figure 6C). With the caveat that the cellular consequences of breaks induced by irradiation may differ substantially from those associated with class switch, our results suggest that the transient IgG secretion in TACI-deficient mice results from Blimp-1 induction by DNA breaks associated with class switch recombination. Thus, in the absence of TACI, as DNA breaks are repaired, Blimp-1 expression may cease, failing to sustain differentiation of long-lived plasma cells, thus causing hypogammaglobulinemia in the long term.
Ionizing radiation induces Blimp-1 expression independently of TACI. (A-B) X-ray irradiation of 18.81 cells (A) or C57BL/6-TACI-ko cells (B) induces γH2AX foci, indicating accumulation of DNA double-strand breaks. Cytospin slides of nonirradiated (0 Gy) or irradiated (4 Gy) cells were stained with an anti-γH2AX antibody (top diagrams) and counterstained with 4,6-diamidino-2-phenylindole (bottom diagrams). (C) Irradiation of 18.81 B cells with 1 Gy or 4 Gy induces Blimp-1 expression transiently, detected by quantitative RT-PCR. Relative expression of Blimp-1 was calculated by determining the ratio Blimp-1/β-actin for each sample. Values reflect 3 independent measurements. (D) Irradiation induces Blimp-1 expression independently of TACI. TACI-ko B cells, isolated from C57BL/6-TACI-ko mice, were irradiated with 4 Gy and cultured with 15 μg/mL of LPS for 24 hours. Blimp-1 expression increased after irradiation of TACI-deficient B cells. RNA was obtained after 24 hours in culture and expression of Blimp-1 or β-actin analyzed by quantitative RT-PCR. Relative expression of Blimp-1 was calculated by determining the ratio Blimp-1/β-actin for each sample. Values reflect 3 independent measurements. Averages were compared by t test. Significant differences were noted: *P < .05, **P < .01, and ***P < .001.
Ionizing radiation induces Blimp-1 expression independently of TACI. (A-B) X-ray irradiation of 18.81 cells (A) or C57BL/6-TACI-ko cells (B) induces γH2AX foci, indicating accumulation of DNA double-strand breaks. Cytospin slides of nonirradiated (0 Gy) or irradiated (4 Gy) cells were stained with an anti-γH2AX antibody (top diagrams) and counterstained with 4,6-diamidino-2-phenylindole (bottom diagrams). (C) Irradiation of 18.81 B cells with 1 Gy or 4 Gy induces Blimp-1 expression transiently, detected by quantitative RT-PCR. Relative expression of Blimp-1 was calculated by determining the ratio Blimp-1/β-actin for each sample. Values reflect 3 independent measurements. (D) Irradiation induces Blimp-1 expression independently of TACI. TACI-ko B cells, isolated from C57BL/6-TACI-ko mice, were irradiated with 4 Gy and cultured with 15 μg/mL of LPS for 24 hours. Blimp-1 expression increased after irradiation of TACI-deficient B cells. RNA was obtained after 24 hours in culture and expression of Blimp-1 or β-actin analyzed by quantitative RT-PCR. Relative expression of Blimp-1 was calculated by determining the ratio Blimp-1/β-actin for each sample. Values reflect 3 independent measurements. Averages were compared by t test. Significant differences were noted: *P < .05, **P < .01, and ***P < .001.
Discussion
Here we show that hypogammaglobulinemia resulting from TACI deficiency results from a primary defect in the differentiation and/or maintenance of long-lived ASCs. Results in this manuscript show, for the first time, that TACI deficiency impairs Blimp-1 expression in B cells responding to protein-based immunizations. Blimp-1 expression decreases clonal expansion in part by inhibiting BCL-6, thus limiting the germinal center reaction. Thus, TACI-deficient animals produce severely blunted IgM responses and hypertrophic germinal centers after immunization and are severely hypogammaglobulinemic. Our findings help to explain the phenotype of CVID resulting from mutations in the TNFRSF13B gene that encodes TACI. Thus, TACI defects impair Blimp-1 expression and consequently decrease differentiation or maintenance of long-lived plasma cells causing hypogammaglobulinemia. Conversely, decreased Blimp-1 expression enhances clonal expansion in response to antigenic stimulation causing lymphoproliferation which, in turn, may increase susceptibility to neoplastic transformation and autoimmunity.
We have previously shown that TACI deficiency impairs antibody responses to polysaccharide antigens, explaining the increased susceptibility to infection with encapsulated organisms of subjects with CVID resulting from TACI mutations.3,6 We and others showed that TACI on B cells facilitates differentiation into ASCs in response to T-independent antigens.6,7 However, whether or not TACI promotes differentiation of ASCs in response to protein-based vaccines has been a matter of controversy. Indeed, some of us and others have previously found apparently normal serum Ig and antibody responses to protein antigens in TACI-deficient mice.3,4 Sakurai et al proposed that TACI may actually inhibit T cell–dependent antibody responses because TACI inhibited Blimp-1 expression and IgG secretion by B cells activated by CD40L.9 In contrast, Castigli et al showed that APRIL stimulated immunoglobulin production induced by anti-CD40 and anti–IL-4 by engaging TACI, suggesting that TACI promotes antibody secretion in response to T cell–dependent signals.8 To understand the mechanisms by which TACI promotes immune fitness and how TACI mutations associated with CVID in human subjects cause hypogammaglobulinemia, we considered 2 possibilities: that TACI induces isotype class switch and therefore production of switched isotypes and/or that TACI promotes differentiation of ASCs.
There is strong evidence to support that engagement of TACI enhances Ig isotype class switch recombination in conjunction with other receptors on B cells. Thus, Castigli et al showed that TACI-deficient B cells produced hardly any class-switched Ig and had decreased Igγ1 and Igϵ germline and Iμ-γ1 and Iμ-ϵ transcripts on stimulation with APRIL and IL-4.19 He et al have recently shown that engagement of TACI triggers Ig class switch recombination by directly activating MyD88 in conjunction with simultaneous stimulation of coreceptors.20 However, because TACI-deficient B cells maintained the ability to class switch in response to stimulation with an anti-CD40 antibody and IL-4 or to LPS and IL-4,19 the results suggested that, although TACI may enhance, it is not absolutely necessary for isotype class switch recombination. Consistent with that conclusion, our results showed robust, albeit transient, IgG production in immunized TACI-deficient animals. Indeed, TACI-deficient mice produced greater amounts of IgG 21 days after immunization with a protein antigen, compared with TACI-proficient mice, indicating that TACI expression by B cells is not necessary to induce isotype class switching to IgG in vivo.
Our results indicated that TACI deficiency primarily impaired differentiation of ASCs rather than isotype class switching. Thus, we found that, despite a reduction in the number of IgG-secreting cells in the spleen, TACI deficiency caused a transient increase in antigen-specific IgG relative to TACI-proficient controls in response to immunization. We propose that extensive germinal center reactions in TACI-deficient animals cause accumulation of DNA breaks that in turn induce Blimp-1 transiently. Transient Blimp-1 expression induces secretion of IgG, even though IgG ASCs do not persist. In agreement with this concept, we showed that DNA double-strand breaks induced by ionizing radiation caused transient expression of Blimp-1, even in the absence of TACI. Because B cells undergoing class switch recombination undergo DNA double-strand breaks,21 B cells expressing class-switched isotypes may initiate antibody secretion as a direct consequence of persisting DNA breaks. However, because continuous Blimp-1 expression is necessary to maintain plasma cell differentiation, transient expression of Blimp-1 is not compatible with long-lived plasma cell survival.15,22,23 Thus, in contrast to TACI-proficient mice, TACI-deficient animals had no long-lived antigen-specific IgG 56 days after immunization.
Our findings indicating that TACI promotes development of ASCs raise the question of how TACI-proficient B cells determine when to differentiate after activation. Two possibilities come to mind. In one, TACI expression or function may change after B-cell activation; in the other, the availability of TACI ligands may increase as B cells grow. In agreement with the idea that TACI expression changes on B-cell activation are studies by Castigli et al8 who showed that stimulation with CD40L increases TACI expression by B cells, and studies by Benson et al24 showing that immunization with protein antigens causes a transient decrease in TACI expression by germinal center B cells, thus precluding premature B-cell differentiation. Regulation of TACI expression may not be the only mechanism controlling differentiation of B cells into ASCs. Indeed, memory B cells in both humans and mice express high levels of TACI24 despite their quiescent state. Consistent with the idea that the availability of TACI ligands determines B-cell differentiation is the work by Chu et al who proposed that activated B cells secrete biologically active B cell–activating factor of the tumor necrosis factor family (BAFF) and/or APRIL, either of which may activate TACI.25 According to Chu et al, germinal center B cells and follicular dendritic cells produce BAFF upon immunization.25 Increasing TACI ligands in the germinal center may thus provide a feedback loop to control clonal expansion by inducing Blimp-1 expression. In addition, TACI ligands may accumulate in certain locations as part of niches fostering differentiation of plasma cells. Thus, Mohr et al showed that the greatest concentration of APRIL was restricted to the ASC-associated myeloid cells colonizing the medullary cords in lymph nodes, suggesting that the local production of these agonists contributes to the early differentiation of ASCs in this location after immunization with alum-precipitated ovalbumin.26
The concept that TACI activity controls B-cell differentiation in a dose-dependent manner predicts that clinical manifestations may occur as a consequence of haploinsufficiency. Indeed, human subjects with CVID resulting from mutations in the TNFRSF13B gene express a nonmutant allele as well. And most of the known mutations do not abolish TACI expression but instead generate receptors that are either deficient in ligand binding and/or have impaired signaling.27-29 In support, Chinen et al showed that haploinsufficiency of the TACI gene results in humoral immune dysfunction in patients with Smith-Magenis syndrome,30 and Lee et al showed that expression of human TACI mutant alleles in mice caused defective antibody production even in the presence of wild-type alleles.31 Which mechanism, control of TACI expression, or the availability of TACI ligands, better explains how TACI regulates B-cell differentiation is incompletely resolved and the subject of present inquiry.
Our results indicating that TACI-induced Blimp-1 provides, for the first time, a molecular and cellular mechanism unifying the key features associated with CVID resulting from deficiencies in TACI. Deficient generation and/or maintenance of ASCs explain the hypogammaglobulinemia and the exquisite vulnerability to infection with encapsulated organisms associated with TACI deficiency. Increased proliferation and accumulation of DNA breaks explain both increased frequency of autoimmune manifestations in TACI-deficient animals5 and in persons with CVID because of TACI deficiency,32 as well as the increased frequency of neoplasia in some CVID patients.1
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Karen Lien who helped with immunochemistry, all the members of our laboratories for comments and criticism, and Ms Jane Bugden for editorial assistance.
This work was supported by the National Institutes of Health (P01 HL079067, J.L.P. and M.C.; and R37 HL052297, J.L.P).
National Institutes of Health
Authorship
Contribution: S.T. performed research, analyzed and interpreted data, and wrote the manuscript; C.C. performed research; R.J.B. contributed vital reagents and analyzed and interpreted data; J.L.P. analyzed and interpreted data and wrote the manuscript; and M.C. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: R.J.B. is listed as a coinventor on patents regarding the TACI gene and protein. The remaining authors declare no competing financial interests.
Correspondence: Marilia Cascalho, Transplantation Biology, University of Michigan, 109 Zina Pitcher Pl, Ann Arbor, MI 48109; e-mail: marilia@umich.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal