Abstract
Characterized by mucocutaneous bleeding arising from a lack of platelet aggregation to physiologic stimuli, Glanzmann thrombasthenia (GT) is the archetype-inherited disorder of platelets. Transmitted by autosomal recessive inheritance, platelets in GT have quantitative or qualitative deficiencies of the fibrinogen receptor, αIIbβ3, an integrin coded by the ITGA2B and ITGB3 genes. Despite advances in our understanding of the disease, extensive phenotypic variability with respect to severity and intensity of bleeding remains poorly understood. Importantly, genetic defects of ITGB3 also potentially affect other tissues, for β3 has a wide tissue distribution when present as αvβ3 (the vitronectin receptor). We now look at the repertoire of ITGA2B and ITGB3 gene defects, reexamine the relationship between phenotype and genotype, and review integrin structure in the many variant forms. Evidence for modifications in platelet production is assessed, as is the multifactorial etiology of the clinical expression of the disease. Reports of cardiovascular disease and deep vein thrombosis, cancer, brain disease, bone disorders, and pregnancy defects in GT are discussed in the context of the results obtained for mouse models where nonhemostatic defects of β3-deficiency or nonfunction are being increasingly described.
Introduction
Glanzmann thrombasthenia (GT) is the most frequently encountered inherited disorder of platelet function.1-3 Patients have a lifelong hemorrhagic syndrome typically characterized by episodes of spontaneous mucocutaneous bleeding. Platelets fail to aggregate in response to stimuli because they lack or have nonfunctional αIIbβ3 integrin (formerly known as GPIIb-IIIa). Resting normal platelets are suspected to have αIIbβ3 in a bent conformation; when platelets are stimulated, the integrin straightens in parallel to the exposure of determinants essential for the binding of fibrinogen (Fg) or other soluble adhesive proteins.3,4 The latter assure aggregation by cross-linking adjacent platelets, a process that cannot occur in GT. Elucidation of this pathway led to the development of integrin-blocking drugs that are strong inhibitors of arterial thrombosis, thereby increasing interest in the clinical manifestations of GT.3,4 Platelet αIIbβ3 also transmits the forces generated by intracellular cytoskeletal proteins during clot contraction and platelet spreading, processes that also fail in patients lacking adequate amounts of functional integrin.
Although the GT phenotype is well defined, bleeding severity differs considerably between affected persons, even within the same family or ethnic group.1,2 In this review, we discuss phenotypic variability, present variant types, and identify possible additional effects associated with β3 deletion, for although αIIb is largely restricted to the megakaryocyte (MK) lineage, β3 is much more widespread in its tissue distribution occurring as αvβ3, the vitronectin receptor.5,6 Data for patients will be compared with those obtained for mouse models of β3 deletion where the absence of αvβ3 influences angiogenesis, reproduction, bone metabolism, inflammation, and cardiovascular disease, among others. Quite clearly, our review establishes the need for a worldwide study for standardized phenotyping of GT across multiple sites.
Bleeding manifestations
Bleeding in GT is primarily mucocutaneous in nature; but although some patients have only minimal bruising, others have frequent, potentially fatal hemorrhages (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Generally, the incidence of bleeding decreases with age. Surgery (including dentistry) has increased bleeding risk, and pregnancy and childbirth represent particularly severe hemorrhagic risks and require follow-up.1,2,7 Platelet transfusion remains the most common treatment, although therapeutic use of recombinant activated factor VII is recommended when isoantibodies induced by prior platelet transfusion are present. Other therapies, including local measures (fibrin sealants, autologous platelet-rich clots), are also available, whereas somatostatin (octreotide) has been reported to be effective for gastrointestinal bleeding (supplemental Table 1). Dissection of platelet and myeloid cell defects by conditional targeting of the β3-integrin subunit in mice has confirmed that platelet deletion of αIIbβ3 alone accounts for the perturbed hemostasis.8 Yet, the reasons why some patients bleed more frequently than others remain elusive.
Causes of αIIbβ3 deficiency in GT
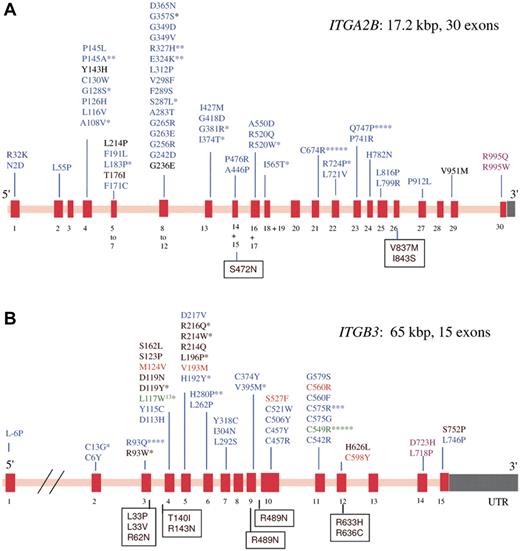
GT has autosomal recessive inheritance, mutations can affect either the ITGA2B or ITGB3 genes that code for αIIb and β3, respectively.9 The genes are closely located at chromosome 17q21.31-32 but are independently expressed. Consanguinity contributes to a greater frequency of GT in some ethnic groups (eg, Iraqi Jews, Palestinian Arabs, French Gypsies). Whereas αIIbβ3 has a high density on normal platelets (up to 100 000 copies), αvβ3 is only minimally present (50-100 copies).10 Figure 1 presents the extensive repertoire of missense mutations located in GT; a panorama of other types of mutation is given in supplemental Figure 1. Large gene deletions are rare, nonsense mutations are abundant as are out-of-frame and in-frame small deletions and insertions. Premature terminations, with or without frameshifts, frequently accompany splicing defects and give truncated proteins and/or mRNA instability. Although mutations occur across both genes, a higher percentage occurs in ITGA2B perhaps because, although smaller, it has more exons (resulting in a higher density of the coding region). Biogenesis of αIIbβ3 occurs in the hematopoietic stem cell and continues throughout MK maturation.11 The αIIb subunit is synthesized with a signal peptide (pro-αIIb); N-linked glycans are attached to pro-αIIb and to β3 in the endoplasmic reticulum where pro-αIIbβ3 complexes form and the signal peptide is removed.12 Further carbohydrate modifications accompany the cleavage of pro-αIIb into mature heavy and light chains in the Golgi apparatus. Mutations in GT can block the production of either subunit or interfere with complex formation and/or trafficking with particular importance given to the αIIb β-propeller region where impaired calcium binding and/or protein malfolding affects trafficking.12-16 If complex formation fails (αIIb with β3; or β3 with αIIb or αv), residual αIIb or β3 undergoes proteosomal degradation. Patients with less than 5% residual αIIbβ3 were originally classified as type I GT, those with 5% to 20% αIIbβ3 type II GT, and rare patients with more than 20% αIIbβ3 but with a qualitative defect preventing function were called variant-type disease. In an early review of the literature, George et al failed to find any correlation between severity of bleeding and GT subtype,1 a conclusion that we show still stands despite the large expansion in the number of reported cases.
Schematic representation showing the spectrum of missense mutations that give rise to GT. From a survey of the literature and on consulting the GT database (http://sinaicentral.mssm.edu/intranet/research/glanzmann), a total of 113 different ITGA2B mutations include 54 missense mutations that distribute across the 30 exons (red bars) of the gene (A), whereas the 72 reported ITGB3 mutations include 44 missense mutations distributed across 15 exons (B). The defects (in single letter amino acid code) responsible for lack-of function variant forms are in black; blue, those that primarily prevent αIIbβ3 expression; green, mutations characteristic of ethnic groups; orange, the substitutions give activated integrin; and rose, GT is associated with macrothrombocytopenia. Asterisks indicate the number of times the defect has been reported in apparently unrelated families. Amino acid substitutions that give rise to human platelet alloantigen systems without affecting αIIbβ3 expression or function are shown in boxes. Mutations giving rise to GT are distributed across both genes. In contrast, variant forms are more likely to have β3 gene defects (see also Table 1).
Schematic representation showing the spectrum of missense mutations that give rise to GT. From a survey of the literature and on consulting the GT database (http://sinaicentral.mssm.edu/intranet/research/glanzmann), a total of 113 different ITGA2B mutations include 54 missense mutations that distribute across the 30 exons (red bars) of the gene (A), whereas the 72 reported ITGB3 mutations include 44 missense mutations distributed across 15 exons (B). The defects (in single letter amino acid code) responsible for lack-of function variant forms are in black; blue, those that primarily prevent αIIbβ3 expression; green, mutations characteristic of ethnic groups; orange, the substitutions give activated integrin; and rose, GT is associated with macrothrombocytopenia. Asterisks indicate the number of times the defect has been reported in apparently unrelated families. Amino acid substitutions that give rise to human platelet alloantigen systems without affecting αIIbβ3 expression or function are shown in boxes. Mutations giving rise to GT are distributed across both genes. In contrast, variant forms are more likely to have β3 gene defects (see also Table 1).
Epidemiologic screening
GT, although a rare disease, has a worldwide distribution. Estimating numbers remains difficult, although in France a recent census by our Center suggests approximately 300 cases, half of which are of gypsy origin. Early mutation analyses mostly identified defects specific for the family under study.9 Only recently has epidemiologic screening begun. D'Andrea et al studied 30 Italian patients and found 21 candidate causal mutations (17 in ITGA2B and 4 in ITGB3).17 Patients with stop codons predicting truncated proteins or those with missense mutations all showed wide differences in bleeding severity. Shortly afterward, 23 new mutations (13 in ITGA2B and 10 in ITGB3) were located in 40 families from Southern India.18 Of these, 20 were novel, with 3 mutations in ITGB3 repeated in multiple families through founder effects. Another group studied 45 more patients from India and identified 22 novel mutations, again with a higher proportion in ITGA2B.19 Analysis of platelet αIIbβ3 showed that 62.2%, 15.5%, and 22.2% of the patients were type I, type II, and variant GT, respectively. Correlations between bleeding severity and genotype or indeed αIIbβ3 expression were not attempted in these later studies.
The capacity of GT platelets to react with fibrin and to store Fg
Agonist-induced soluble Fg binding is mediated by a KQAGDV sequence at the C-terminus of the 2 fibrinogen γ-chains, although RGD sequences are also able to bind to αIIbβ3.3 But defects of αIIbβ3 in GT can also affect clot retraction and the uptake of Fg into α-granules. Studies with the antiplatelet drug abciximab showed that αIIbβ3 recycles between external and internal pools.20 In type II or variant GT, receptor recycling associated with Fg deposition can lead to near-normal amounts of α-granule Fg. For example, a type II French patient with a Cys598Tyr mutation in β3 and approximately 10% αIIbβ3 expression had more than 60% of the normal α-granule Fg content.21 The fact that some mutations (eg, ITGB3 Ser752Pro) allow Fg trafficking to α-granules and a normal clot retraction (Table 1; see “Variant-type GT”) while abrogating activation-dependent soluble Fg binding and aggregation suggests gaps in our knowledge on integrin function. Functional studies suggest further heterogeneity, platelets from the aforementioned type II French patient, and 2 French patients with variant GT (ITGB3, Ser752Pro; ITGB3 Arg214Trp), although unable to bind Fg, adhered and formed small thrombi when their blood was perfused through collagen-coated capillaries.22 A thick mesh composed of VWF and fibrin surrounded the thrombi, suggesting residual shear-dependent platelet reactivity. In contrast, thrombi were not observed for 2 GT patients totally lacking αIIbβ3. But such phenotypic differences did not correlate with bleeding history.
Landmark cases contributing to the clinical and phenotypic heterogeneity given by ITGA2B and ITGB3 mutations
| Mutation . | Bleeding . | Aggregation . | Clot retraction . | Platelet Fg . | αIIbβ3 expression/activation state . | Comments . |
|---|---|---|---|---|---|---|
| β3 D119Y | Severe | Absent | Absent | None | Normal/NA | Identification of an Fg binding site |
| β3 R214Q or W | Severe | Absent | Absent/low | None/low | Normal/NA | Unstable integrin/altered Ca2+ binding |
| β3 S162L | Severe | Absent | Normal | NS | 16%-27%/NA‡ | Refractory integrin poorly expressed |
| β3 L262P | Moderate | Absent | Substantial | NS | 30%/NA | Unstable integrin poorly expressed |
| β3 S752P* | Mild | Absent | Substantial | Normal | 50%/NA | Signaling role of β3 cytoplasmic tail |
| β3 R724ter* | Severe | Absent | NS | NS | 35%-50%/NA | Signaling role of β3 cytoplasmic tail |
| αIIb Y143H | Mild | Absent | Substantial | NS | 36%-41%/NA | Altered residual β-propeller domain |
| αIIb T176I | Severe | Absent | Much reduced | None | 24%/NA | Altered residual β-propeller domain |
| β3 S527F | Mild | Absent | NS | NS | NR/PAC-1 and Fg§ | αIIbβ3 restrained in active conformation |
| β3 C549R | Severe | Absent | Absent | NS | 1%-14%/PAC-1§ | Partially activated residual integrin |
| β3 C560R | Moderate | Much reduced | Much reduced | Substantial | 20%/PAC-1 and Fg | Fully activated residual integrin |
| αIIb R995Q* or W† | Mild | Reduced | Present | Substantial | 15% and 50%-70%/PAC-1 | Thrombocytopenia and anisocytosis |
| β3 D723H† | None | Normal | NS | NS | Normal/PAC-1 | Thrombocytopenia and anisocytosis |
| β3 L718P | Severe | Much reduced | NS | NS | 47%-78%/PAC-1‖ | Aggregation and secretory defects (P-selectin) + thrombocytopenia |
| β3 c.2134 + 1G > C + 40aa deletion† | Severe | Much reduced | Normal | NS | 32%-66%/NA | Aggregation defect + thrombocytopenia + large platelets |
| Mutation . | Bleeding . | Aggregation . | Clot retraction . | Platelet Fg . | αIIbβ3 expression/activation state . | Comments . |
|---|---|---|---|---|---|---|
| β3 D119Y | Severe | Absent | Absent | None | Normal/NA | Identification of an Fg binding site |
| β3 R214Q or W | Severe | Absent | Absent/low | None/low | Normal/NA | Unstable integrin/altered Ca2+ binding |
| β3 S162L | Severe | Absent | Normal | NS | 16%-27%/NA‡ | Refractory integrin poorly expressed |
| β3 L262P | Moderate | Absent | Substantial | NS | 30%/NA | Unstable integrin poorly expressed |
| β3 S752P* | Mild | Absent | Substantial | Normal | 50%/NA | Signaling role of β3 cytoplasmic tail |
| β3 R724ter* | Severe | Absent | NS | NS | 35%-50%/NA | Signaling role of β3 cytoplasmic tail |
| αIIb Y143H | Mild | Absent | Substantial | NS | 36%-41%/NA | Altered residual β-propeller domain |
| αIIb T176I | Severe | Absent | Much reduced | None | 24%/NA | Altered residual β-propeller domain |
| β3 S527F | Mild | Absent | NS | NS | NR/PAC-1 and Fg§ | αIIbβ3 restrained in active conformation |
| β3 C549R | Severe | Absent | Absent | NS | 1%-14%/PAC-1§ | Partially activated residual integrin |
| β3 C560R | Moderate | Much reduced | Much reduced | Substantial | 20%/PAC-1 and Fg | Fully activated residual integrin |
| αIIb R995Q* or W† | Mild | Reduced | Present | Substantial | 15% and 50%-70%/PAC-1 | Thrombocytopenia and anisocytosis |
| β3 D723H† | None | Normal | NS | NS | Normal/PAC-1 | Thrombocytopenia and anisocytosis |
| β3 L718P | Severe | Much reduced | NS | NS | 47%-78%/PAC-1‖ | Aggregation and secretory defects (P-selectin) + thrombocytopenia |
| β3 c.2134 + 1G > C + 40aa deletion† | Severe | Much reduced | Normal | NS | 32%-66%/NA | Aggregation defect + thrombocytopenia + large platelets |
Variants were included on the basis of their historical importance and of the availability of clinical and biologic data. Mutations are referenced in “Variant-type GT.” Data are included for platelet aggregation, clot retraction, platelet Fg, and platelet surface αIIbβ3 expression and its activation status. When a range of αIIbβ3 expression is given, it corresponds to that given by different monoclonal antibodies. For platelet activation, a positive response for PAC-1 and/or Fg binding is specified. Bleeding severity was noted as described in the original reports or case histories. Mild bleeding basically refers to few spontaneous or trauma-related bleeding manifestations that are easily controlled; moderate bleeding covers frequent epistaxis and/or cutaneous manifestations, infrequent oral cavity and/or gastrointestinal bleeding, menorrhagia controlled by contraceptive pills; severe bleeding includes epistaxis requiring packing, cauterization, and/or blood transfusion and trauma-related bleeding requiring hospitalization and blood transfusion or alternative therapies, spontaneous or trauma-related severe bleeding.
NS indicates not studied; NA, no activation with physiologic agonists; and NR, not reported for platelets.
Heterozygous mutations associated with null alleles.
Only for 3 mutations was transmission recognized as autosomal dominant (AD).
Only the absence of conformational change to RGD peptide binding was studied.
Studied in transfected heterologous cells only.
Decreased in platelets but spontaneously active in transfected cells, effects linked to integrin clustering.
Integrin αvβ3 in thrombasthenia
Platelets of Israeli-Arabs with an ITGA2B mutation (c.IVS3(-3)-418del + frameshift) preventing αIIbβ3 expression had a 2-fold increase in αvβ3. Platelets of Iraqi-Jewish patients with an ITGB3 mutation (c.2031-2041del/premature termination) lacked both integrins but contained 5-fold more vitronectin while still not storing Fg.10,23,24 It was proposed that vitronectin, synthesized in MKs, is normally transported out of the cells by αvβ3 largely localized to small vesicular structures in platelets.25 Nevertheless, clinically, the Iraqi-Jewish and Arab patients are indistinguishable.26 Some β3 mutations (eg, Leu196Pro and His280Pro, a mutation prevalent in Japan) primarily affect αIIbβ3 but not αvβ3 expression.27-29 Ligand binding to the integrins may also be differentially impaired; for example, αIIbβ3196Pro supported CHO cell spreading on Fg, but αvβ3196Pro did not and the difference extended to clot retraction. As αvβ3 is expressed in endothelial cells, osteoclasts, and smooth muscle cells, among others, such results imply that the effect of β3 amino acid substitutions on these cells must be assessed individually.
Variant-type GT
Obligate heterozygotes for GT have platelets with intermediate amounts of αIIbβ3 but normal platelet function, and they do not bleed.1,2 In variant-type GT, patients express αIIbβ3 at levels that would normally allow platelet aggregation, but amino acid substitutions render the integrin nonfunctional. Variants are diagnosed through the inability of their platelets to bind labeled Fg or antibodies recognizing activation-dependent determinants on αIIbβ3.30 The nature and position of the mutation define the residual functional response. Significantly, most of them concern ITGB3. Table 1 summarizes the platelet characteristics of the most studied cases of variant GT.
Mutations affecting extracellular domains of β3
The first variant to be characterized was from Guam and possessed a homozygous Asp119Tyr substitution in β3 (Figure 2).31 This occurs within the β3 I-like domain and interferes with the function of αIIbβ3 and αvβ3 by inducing the loss of a divalent cation (Mg2+) structured Fg-binding site within the metal ion-dependent adhesion site (MIDAS).4 Three unrelated patients (2 from France and 1 from Australia; all with moderate to severe bleeding, Table 1) possessed homozygous substitutions of Arg214 (Arg214Gln or Trp) in the ADMIDAS (adjacent to MIDAS) Ca2+-binding domain of β3 (Figure 2).32-37 These substitutions made αIIbβ3 unstable, sensitive to divalent-cation chelation, and unable to bind Fg; they not only prevent aggregation, they also stop clot retraction and platelet Fg capture, reinforcing the idea that αIIbβ3 is required for each task. Although 3-dimensional structures of the unactivated and activated (Fg-binding) forms of αIIbβ3 have been defined and key residues essential for Fg binding located on both the β3 MIDAS and ADMIDAS domains,3,4,36,37 different structural requirements may be required for clot retraction and Fg trafficking. This is underlined by a homozygous β3 Ser162-> Leu mutation situated near the synergistic metal binding site (SyMBS; Figure 2) in a patient whose platelets fail to aggregate but undergo clot retraction.38 Platelet expression of αIIbβ3 was assessed as 16% to 27%, but discrepant monoclonal antibody binding suggested altered complex-dependent determinants, a situation repeated for a β3Leu262Pro substitution.39 A β3 Arg93Gln mutation also supported clot retraction but not aggregation; significantly, a patient with homozygous expression had a milder bleeding syndrome and greater αIIbβ3 expression than a second patient who associated the variant allele with a null allele.18 A homozygous Ser123Pro substitution was detected in β3 in an Indian girl with the clinical signs of GT but normal platelet αIIbβ3 expression.40 Paradoxically, ADP-induced Fg binding still occurred despite a lack of aggregation to ADP and collagen, a finding that needs further explanation.
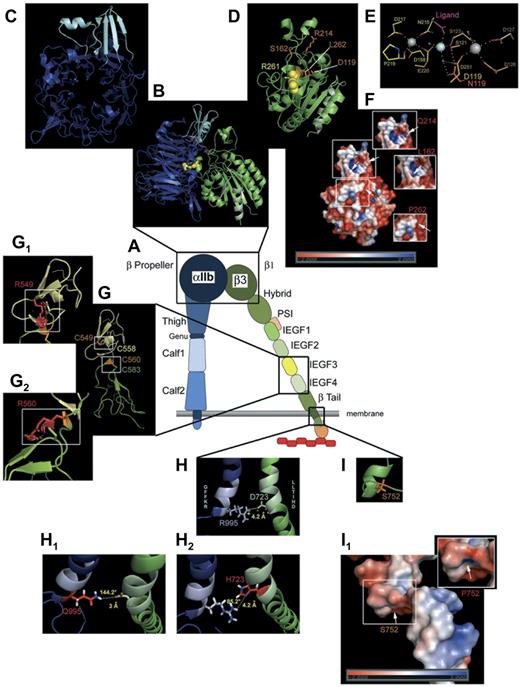
Modeling of selected mutations on αIIbβ3 structure. (A) Schematic representation of αIIbβ3 in open conformation. (B) Computer-drawn ribbon diagram of αIIb (blue, cap in light blue) and β3 (green) headpieces in open conformation; Arg261 (yellow spheres) in β3 is shown as a space-filling model.29 A 90° rotation of the αIIb β-propeller (C) and the β3 βΑ I-like domain (D) exposes the surfaces normally in contact with each other. (D) Amino acids (orange sticks) affected by mutations giving variant forms (Table 1). (E) Metal coordination sites in the SyMBS (yellow), MIDAS (orange), and ADMIDAS (brown) domains; N atoms are blue and O atoms are red; Ca2+ (gray spheres), Mg2+ (pale blue spheres), and water (blue small spheres). Metal coordination and hydrogen bonds are shown as dashed lines; Asp119 (orange) and the mutated Asn (red) are superimposed. E220 contributes to both SyMBS and MIDAS, although colored yellow. (F) Electrostatic potential surfaces of the β1 domain, with views (small windows) of charge changes caused by the 3 highlighted mutations (for clarity, the corresponding mutation sites are indicated on the whole image by white arrows). (G) Ribbon diagrams of I-EGF3 (yellow) and I-EGF4 (green) domains of β3. Selected disulfides are illustrated with Cys549 (orange stick) interacting with Cys558 (yellow stick), and Cys560 (orange stick) bonded to Cys583 (green stick).55 Mutations (G1, G2) are shown as graphical red stick representations in small windows; graphical “bumps” (red discs) indicate steric interactions caused by the Arg549 and Arg560 substitutions. (H) Ribbon diagrams of the cytoplasmic domains of αIIbβ3. Shown is the ionic interaction of Arg995 of αIIb (pale blue stick, positively charged) and Asp723 of β3 (pale green stick, negatively charged). (H1-H2) Amino acid changes in the salt link in cytoplasmic domain variants (Table 1). (H1) Replacement of Arg995 of αIIb by Gln modifies the ionic interaction with a hydrogen bond of moderate force with respect to the distance and angle formed by the 3 atoms engaged in the link. (H2) Substitution of Asp723 of β3 by His causes the complete loss of the interaction with Arg995. (I) Ser752 (orange stick) of β3 with (I1) showing images of the electrostatic potential surface changes induced by the mutation Ser752Pro. Models were obtained using the PyMol Molecular Graphics System Version 1.3 (Schrödinger LLC; www.pymol.org) and 2vdo, 3fcs, and 2knc pdb files for crystal structure of αIIb and/or β3 subunits. Amino acid changes are rotamer incorporated from the Dunbrack Backbone library with the maximum probability. Electrostatic potential surface calculation and visualization were obtained with the Adaptive Poisson-Boltzmann Solver software incorporated in PyMol Version 1.3 software.104
Modeling of selected mutations on αIIbβ3 structure. (A) Schematic representation of αIIbβ3 in open conformation. (B) Computer-drawn ribbon diagram of αIIb (blue, cap in light blue) and β3 (green) headpieces in open conformation; Arg261 (yellow spheres) in β3 is shown as a space-filling model.29 A 90° rotation of the αIIb β-propeller (C) and the β3 βΑ I-like domain (D) exposes the surfaces normally in contact with each other. (D) Amino acids (orange sticks) affected by mutations giving variant forms (Table 1). (E) Metal coordination sites in the SyMBS (yellow), MIDAS (orange), and ADMIDAS (brown) domains; N atoms are blue and O atoms are red; Ca2+ (gray spheres), Mg2+ (pale blue spheres), and water (blue small spheres). Metal coordination and hydrogen bonds are shown as dashed lines; Asp119 (orange) and the mutated Asn (red) are superimposed. E220 contributes to both SyMBS and MIDAS, although colored yellow. (F) Electrostatic potential surfaces of the β1 domain, with views (small windows) of charge changes caused by the 3 highlighted mutations (for clarity, the corresponding mutation sites are indicated on the whole image by white arrows). (G) Ribbon diagrams of I-EGF3 (yellow) and I-EGF4 (green) domains of β3. Selected disulfides are illustrated with Cys549 (orange stick) interacting with Cys558 (yellow stick), and Cys560 (orange stick) bonded to Cys583 (green stick).55 Mutations (G1, G2) are shown as graphical red stick representations in small windows; graphical “bumps” (red discs) indicate steric interactions caused by the Arg549 and Arg560 substitutions. (H) Ribbon diagrams of the cytoplasmic domains of αIIbβ3. Shown is the ionic interaction of Arg995 of αIIb (pale blue stick, positively charged) and Asp723 of β3 (pale green stick, negatively charged). (H1-H2) Amino acid changes in the salt link in cytoplasmic domain variants (Table 1). (H1) Replacement of Arg995 of αIIb by Gln modifies the ionic interaction with a hydrogen bond of moderate force with respect to the distance and angle formed by the 3 atoms engaged in the link. (H2) Substitution of Asp723 of β3 by His causes the complete loss of the interaction with Arg995. (I) Ser752 (orange stick) of β3 with (I1) showing images of the electrostatic potential surface changes induced by the mutation Ser752Pro. Models were obtained using the PyMol Molecular Graphics System Version 1.3 (Schrödinger LLC; www.pymol.org) and 2vdo, 3fcs, and 2knc pdb files for crystal structure of αIIb and/or β3 subunits. Amino acid changes are rotamer incorporated from the Dunbrack Backbone library with the maximum probability. Electrostatic potential surface calculation and visualization were obtained with the Adaptive Poisson-Boltzmann Solver software incorporated in PyMol Version 1.3 software.104
β3 cytoplasmic domain mutations
Platelets from a South American man with a mild bleeding history expressed 50% αIIbβ3 but failed to aggregate or bind Fg when stimulated. Nevertheless, his platelets supported clot retraction and possessed a normal α-granule pool of Fg (Table 1).41,42 A heterozygous Ser752Pro substitution in the β3 cytoplasmic tail (Figure 2) accounted for the defect and was said by the authors to be associated with a null allele on the basis that his daughter's platelets, which also expressed 50% αIIbβ3, aggregated normally but did not possess the β3 cytoplasmic domain mutation.41 In modifying β3 intracytoplasmic domain structure (Figure 2), Pro752 not only abrogates αIIbβ3 and αvβ3 activation, it also blocks “outside-in signaling” and cell spreading after integrin engagement on surface-bound adhesive proteins.42-45 More precisely, the mutation prevents the binding to β3 of kindlin-3, a cytoplasmic protein required for integrin activation (for more information on kindlin-3, see “LAD-3 syndrome”).46 A similar platelet phenotype was reported for a black American child shown to be compound heterozygote with a nonexpressed allele and platelets with intermediate amounts of αIIbβ3 containing a truncated β3 with only 8 membrane proximal amino acids of the 47 that compose the cytoplasmic tail and therefore lacking multiple active sites, including those responsible for talin binding.47 Significantly, this patient had a more severe bleeding history than the patient with the more specific Ser752Pro mutation. CHO cells expressing αIIbβ3Arg724ter failed to spread on Fg, confirming defective “outside-in” integrin signaling and showing absent FAK phosphorylation.47 For this patient, drug/antibody complexes reacting with αIIbβ3Arg724ter failed to activate FcγRIIA, implying that β3 has trans-signaling properties.48
Mutations affecting αIIb
Proportionally fewer mutations of ITGA2B give rise to variant GT (Figure 1; Table 1). One example is a heterozygous Cys674Arg substitution in αIIb that disrupts the Cys674-Cys687 disulfide; it gave platelets with 30% αIIbβ3 when associated with a null allele.49 Association and trafficking of the subunits were retarded because of an abnormal interaction of αIIbArg674 with the BiP chaperone. A Japanese variant (KO mutant) with a homozygous 6-bp insertion causing the addition of Arg-Thr within the Cys146-Cys167 loop of αIIb had platelets fully expressing αIIbβ3 unable to bind Fg or PAC-1 when activated.50 Alanine-scan mutagenesis of oxygenated residues within this loop confirmed Asp163 as critical for ligand binding. Similarly, a heterozygous Tyr143His substitution in αIIb combined with the null expression of the second allele gave approximately 40% levels of αIIbβ3 that failed to bind PAC-1 or Fg when activated.51 Again, the response depended on the mutation; heterologous cells expressing the KO mutant or αIIbAla163β3 failed to adhere to Fg, whereas cells expressing αIIbHis143β3 showed residual cell attachment and spreading. The KO and Asp163Ala mutations blocked clot retraction, whereas cells expressing His143 partially retracted the clot. Occasional mutations in αIIbβ3 also prevent Fg trafficking to α-granules. An example is a homozygous Thr176Ile substitution in the W3:1-2 connecting strand of the β-propeller of αIIb.52 The β-propeller domain at the N-terminus of the αIIb subunit is of critical importance for αIIbβ3 biogenesis; it is the site of many mutations giving rise to type I and type II GT.14,16
Activating mutations in β3
The first evidence for up-regulated integrins came when an artificially induced Thr562Gln mutation in the extracellular region of β3 gave rise to activated αIIbβ3 and αvβ3 in heterologous cells.53 Recently, a heterozygous Ser527Phe substitution in the I-EGF-3 domain of β3 of a GT patient was predicted to hinder adoption of the bent resting conformation of αIIbβ3 and the mutated integrin spontaneously bound Fg and the PAC-1 antibody when expressed in CHO cells (Table 1).54 But most activating mutations involve breakage of disulfide bonds within the β3 EGF domains where the cysteine-rich core confers a structural constraint on the receptor.55 Mostly, they severely limit αIIbβ3 expression; this was the case for a Cys549Arg mutation in β3 in 6 Jordanian families with severe GT that disrupted a conserved disulfide between Cys549 and Cys588 (Figure 2).56 Nevertheless, production of recombinant integrin in BHK cells confirmed that β3549Arg led to constitutively active αIIbβ3 (PAC-1 binding). However, for these patients, low αIIbβ3 levels in the platelets probably accounted for the bleeding phenotype. Mutations that allowed a greater expression of spontaneously active αIIbβ3 on platelets include Cys560Arg (or Phe) and Cys598Tyr (Figure 2).18,57 The gain-of-function Cys560Arg mutation was detected in a French man with mild bleeding, moderate thrombocytopenia, and a history of kidney disease. It allowed approximately 20% surface αIIbβ3 expression. Platelet aggregation and clot retraction were much reduced but not absent; platelet Fg content was normal. Platelets spontaneously bound antibodies to activation-dependent epitopes on αIIbβ3, whereas immunoelectron microscopy highlighted the presence of Fg on what were otherwise unactivated platelets ex vivo. Blocking of the integrin with Fg was speculated to contribute to the lack of function.57 Interestingly, in a preliminary report, a “knock-in” mouse model with platelets possessing αIIbArg560β3 was associated with high mortality, thrombosis, hemorrhage, and necrosis.58 Substitution of Cys560 by any of 12 different amino acids always led to activated αIIbβ3; thus, breaking the disulphide was the crucial event.55,57 A heterozygous β3 Met124Val substitution 1 residue downstream from the MIDAS core occurred in combination with a second heterozygous inactivating β3 mutation. The patient's platelets failed to bind Fg or PAC-1 for β3 Met124Val strongly interfered with αIIbβ3 biosynthesis and/or expression. Yet this mutant integrin showed some degree of activation when expressed in CHO cells, which aggregated spontaneously in the presence of Fg. This study therefore reinforced the view that a noncysteine mutation could also be potentially activating; it was hypothesized that receptor clustering caused an activated state by increasing integrin avidity for Fg.59
Macrothrombocytopenia in patients with ITGA2B and ITGB3 mutations
Although the majority of patients with mutations in ITGA2B and ITGB3 have a normal platelet size and count, this is not always the case. This was first shown for an Italian boy with mild bleeding whose platelets aggregated minimally to ADP but gave a slow, irreversible aggregation to thrombin.60,61 Levels of αIIbβ3 were at the lower limit of those able to support aggregation. Moderate thrombocytopenia and anisocytosis with large platelets were linked to a paternally inherited heterozygous Arg995Gln substitution within the GFFKR sequence of the cytoplasmic domain of αIIb (Figure 2). PAC-1 binding to a stably transfected CHO cell line showed the mutated integrin to be partially activated; nonetheless, it failed to spontaneously bind Fg. A maternally inherited heterozygous intronic deletion (c1440-13_1440-1del) within the splicing acceptor for exon 15 led to an absent expression of the second allele for αIIb and largely accounted for the low αIIbβ3 content.62 The subsequent observation that a heterozygous ITGA2B Arg995Trp mutation in 5 patients from 3 Japanese families gave a similar phenotype made it unlikely that a coexpressed second mutation accounts for the macrothrombocytopenia.63 In the Japanese families, a normal second allele meant that surface αIIbβ3 levels were 50% to 70% of controls. Spontaneous binding of PAC-1 to platelets and studies on αIIb995Trpβ3-transfected 293T cells confirmed the mutated integrin to be partially activated (ie, to have undergone conformational changes allowing PAC-1 access to its binding site). Cytoplasmic domain residues αIIbArg995 and β3Asp723 form a salt bridge, and breaking this clasp is thought to be a key step in integrin activation.64 Significantly, macrothrombocytopenia, but without a platelet aggregation defect (thereby showing that β3 mutations can extend beyond the GT phenotype), characterized 5 members of a family with a heterozygous partially activating Asp723His substitution in β3, a substitution that has a considerable charge effect.65 Furthermore, abnormal proplatelet formation was shown in MKs cultured in vitro from the propositus's CD34+ stem cells. Unusually, when transfected with αIIbβ3His723, CHO cells spontaneously formed proplatelet-like protrusions when plated on Fg, a finding reproduced for CHO cells transfected with αIIb995Trpβ3.63
In 2 Italian families, severe mucocutaneous bleeding, moderate thrombocytopenia, and large platelets with defective aggregation were inherited as an autosomal dominant trait (Table 1).66 Molecular analysis revealed a heterozygous in-frame deletion causing loss of amino acids 647 to 686 from the transmembrane domain of β3. A dominant negative effect was hypothesized. The result was a low surface expression of αIIbβ3, but PAC-1 binding was not significantly enhanced. Platelet spreading on Fg was additionally impaired as was shear-dependent platelet adhesion to collagen. Clot retraction was normal. A heterozygous Leu718Pro mutation in the membrane-proximal region of the β3 cytoplasmic domain of a Spanish woman also led to a severe bleeding phenotype and macrothrombocytopenia.67 Platelet aggregation was much reduced to all agonists, and this time there was a secretion defect with little surface expression of P-selectin and CD63. Again, the surface expression of αIIbβ3 was selectively reduced, and platelets showed impaired spreading and defective lamellipodia formation on Fg. Yet CHO cells expressing recombinant αIIbβ3Pro718 directly bound Fg and PAC-1 and, when plated on Fg, they also formed long extensions. Although experiments on CHO cells should be treated with caution in view of their fibroblastic origin, the aforementioned results globally suggest that changing the affinity status of αIIbβ3 influences cell reactivity with adhesive proteins. So far, mutations associated with changes in platelet size cluster near or in the membrane. Changing the activation state and/or αIIbβ3 avidity may lead to an altered αIIbβ3 signaling; an altered megakaryocyte maturation and/or timing of proplatelet formation may be the key, although the precise mechanisms require further elucidation.
LAD-3 syndrome
In leukocyte adhesion deficiency (LAD) syndromes, mutations with loss of function of β2 integrins on leukocytes (LAD-1) or altered fucosylation of glycoproteins (LAD-2) give rise to immune-deficiency syndromes associated with a defective inflammatory response and infections. In LAD-3, patients associate a greater bleeding severity (including intracerebral bleeding) with immune deficiency and recurrent infections but with a normal expression of β1, β2, and β3 integrins.68,69 These patients have a defect common to the “inside-out” signaling pathways of the integrins. Infections range from bacterial pneumonia and early septicemia to fungal disease; osteopetrosis in some patients indicates abnormal osteoclast function. Initial studies implied that LAD-3 patients were deficient in a guanine nucleotide exchange factor, CALDAG-GEFI, and that this led to decreased Rap1 activation.70 However, mutations in FERMT3 (encoding kindlin-3) were later found to account for the phenotype.46,71-74 Kindlin-3 belongs to a family of proteins that cooperate with talin in inside-out integrin activation.73 Possessing a single FERM (F) domain with 3 subdomains, kindlin-3 binds through its third subdomain (F3) to the membrane distal NXXY motif of the β-integrin cytoplasmic tail; this differs from talin, which binds to the membrane proximal NXXY motif.73 Significantly, kindlin-3 binding was abrogated by the GT variant β3 Ser752Pro mutation (see “Variant-type GT”), again raising questions as to his mild bleeding phenotype.71 Stop codons or splicing defects leading to truncation and/or nonexpression of kindlin-3 predominate in LAD-3 patients.46,71-74 A Gly308Arg change in FERM subdomain 2 of 1 patient affected migration of B cells but not adhesion, leaving open the possibility that missense mutations can give rise to mild variant forms of LAD-3. In a recent development, Bialkowska et al reported that kindlin-3 played a role in αvβ3-mediated adhesion of endothelial cells.75 Defective endothelial cell function may contribute to the increased bleeding in LAD-3 patients; an involvement of other integrins in endothelial cell adhesion may explain the more pronounced bleeding phenotype compared with patients lacking β3.
SNPs and GT phenotype
Studies assessing whether certain single nucleotide polymorphisms (SNPs) favor bleeding or by increasing fibrin formation result in a milder form of the disease in GT are just beginning. D'Andrea et al screened 25 Italian GT patients but found no evidence for an increased number of thrombophilic FV Leiden and prothrombin A20210 mutations.76 However, ten Cate et al reported a 48-year-old man with type I GT with factor V Leiden who, on 3 occasions, developed deep vein thrombosis (DVT) in a leg.77 A survey of 41 Indian GT patients revealed 4 with at least 1 human platelet alloantigen-1b allele and a milder form of the disease, although the presence of more than 10% aggregation to ADP and residual αIIbβ3 could alone explain this finding.78 Although the human platelet alloantigen-1b allele has a low gene frequency of 0.15 in whites, we noted that it predominated in French gypsies with GT and a moderate to severe bleeding phenotype.79 These patients have a G > A substitution at the splice donor site of intron 15 in ITGA2B (supplemental Figure 1) and platelets totally lacking αIIbβ3. The frequency of major SNPs on GPIbα, GPVI, or the α2 integrin subunit have not shown consistent changes in expression in the small GT cohorts so far examined76,79 ; nonetheless, a much larger study is warranted in view of the wide range of expression of these adhesion receptors on platelets of normal donors.80
Frequency of major pathologies associated with GT
A number of major pathologies have been reported in GT patients, although their frequency and link to the genetic defect causative of GT have yet to be adequately evaluated. Supplemental Table 2 summarizes well-documented cases. Reports of brain disorders are confined to a Moroccan girl who combined GT (and major bleeding) with tuberous sclerosis. Although molecular analysis identified a homozygous Cys542Arg substitution in β3, other GT patients with unpaired cysteines in β3 fail to show neurologic defects, and genes associated with tuberous sclerosis are located on chromosomes 9 and 16. Major brain disorders, such as Alzheimer disease, have not been reported in GT. The purported role for αvβ3 in bone resorption prompted Coller et al to measure bone density in women from the Iraqi-Jew GT cohort lacking β3.26 Although clear evidence for bone thickening was not obtained, the possibility was not ruled out (also see “Mouse models with deficiencies of αIIbβ3 and αvβ3”). Infantile malignant osteopetrosis in a Turkish boy with a noncharacterized variant form of GT is suggestive of kindlin-3 deficiency (see “LAD-3 syndrome”). GT in a German woman with developmental defects, including skeletal abnormalities, was associated with compound heterozygosity for Arg358His and Gly412Arg substitutions in αIIb (not expressed in bone cells).
It is reasonable to ask whether metastasis and cancer development are modified in GT for normal platelets to facilitate cancer cell adhesion to subendothelium and metastasis while αvβ3 is involved in tumor angiogenesis.81 Several reports have featured surgical removal of carcinoma in GT patients (supplemental Table 2). In Bordeaux, an elderly type II GT male patient (with ∼ 10% surface αIIbβ3 expression, a lack of platelet aggregation, subnormal clot retraction, and moderate bleeding syndrome) died from the consequences of lung cancer with brain metastasis (P. Nurden, unpublished observations, May 2011). Xeroderma pigmentosum, an autosomal recessive condition characterized by severe sun sensitivity, early skin cancers, and abnormal DNA repair, occurred in a 12-year-old Turkish girl with GT. Acute myeloid leukemia was described in a Japanese GT patient from Hiroshima with a novel point mutation in ITGA2B, in whom bone marrow transplantation from a HLA-matched donor was successfully performed.
Atherosclerosis and coronary artery disease have also been reported in GT (see supplemental Table 2 for details). The most comprehensive study is the screening of 7 Israeli GT patients (46-66 years of age) belonging to the Arab (lacking αIIbβ3) or the Iraqi-Jew cohorts (lacking both αIIbβ3 and αvβ3) for early signs of atherosclerosis. An increased intima/media thickness and atherosclerotic plaques in 5 of them confirmed that atherosclerosis can occur. Several isolated reports also describe cardiac or ischemic problems in GT patients (detailed in supplemental Table 2). However, these need to be assessed with caution in view of the absence of mutation screening. Overall, it is difficult to assess incidence of cardiovascular disease in GT in view of the young age of many patients.
Interestingly, several cases of DVT have been reported in patients with GT (detailed and referenced in supplemental Table 2). As well as the previously discussed factor V Leiden-positive Dutch patient (see section on SNPs and GT phenotype),77 DVT has been described in at least 3 other patients, including the South American man with a Ser752Pro mutation in the β3 cytoplasmic tail (see section on GT-type variants). None of these latter patients had known risk factors for venous thrombosis. Again, it is worthy to underline the presence of the Ser752Pro variant with absent platelet aggregation and a mild bleeding type, despite abrogation of kindlin-3 binding to the β3 cytoplasmic tail.
Other topics worthy of discussion include inflammation and fertility. The proinflammatory sCD40L, released from platelets in an αIIbβ3-dependent manner, is markedly reduced in type I GT.82 A Toll-like receptor 2-integrin β3 complex senses bacterial lipopeptides with an important role for vitronectin and αvβ3.83 Monocytes from patients lacking β3 integrins were unresponsive to bacterial lipopeptides with impaired cytokine production. Yet infections and an altered inflammatory response are not recognized problems in GT. Coller at al26 looked for evidence of infertility within the Iraqi-Jewish GT cohort lacking αvβ3; 3 of 5 women who attempted to become pregnant succeeded. Secondary amenorrhea resulting from repeated ovarian hemorrhage was suggested to contribute to the fertility problem. An unexpected low number of pregnancies and a high frequency of spontaneous abortions have been reported in GT women in a large epidemiologic study in Iran.84 The consequences of the absence of αIIbβ3 and αvβ3 in ovulation as well as placental and fetal development require further clarification.
Mouse models with deficiencies of αIIbβ3 and αvβ3
Transgenic mouse models offer a clearer picture of the influence of integrin deletion in nonhemostatic processes. Although mice are not humans and differences between clinical findings in patients and mice may be a reflection of species differences, it is worthwhile to look at the studies on β3- and αIIb-deficient mice. The first report of β3-integrin-deficient mice underlined that, although they reproduced the GT bleeding phenotype, there were diverse embryonic and postnatal changes.85 Placental defects were seen and these led to an increased fetal mortality, a finding suspected but as yet unproven in human GT (see “Frequency of major pathologies associated with GT”). Unexpectedly, mice lacking β3 not only supported tumorigenesis, tumor growth was actually improved.86 Enhanced angiogenesis in the β3 null mice was linked to an unexpected increase in the platelet storage pool of VEGF and elevated levels of VEGF receptor-2 (Flk-1) on endothelial cells.87 Enhanced Flk1 signaling allowed increased endothelial cell migration and proliferation in response to VEGF as well as greater VEGF-mediated permeability.88
Enigmatically, coronary capillaries fail to mature in male but not female β3-null mice.89 Its correction by blocking VEGF implies that αvβ3 plays a critical role in coronary vascular development and the vascular response to VEGF. Cardiac hypertrophy (with systolic and diastolic dysfunction) and accelerated atherosclerosis were also noted in β3-deficient mice.90 An increased macrophage infiltration in β3−/− cardiac tissue was associated with a greater expression of inflammatory genes, such as those encoding IL-1β, IL-6, and TNF-α.91 When apoE-null and LDL receptor-null mice were transplanted with β3-deficient marrow, they showed increased atherosclerosis and a higher mortality with Western-type high fat feeding. Multiple knockouts obtained by crossing β3−/− mice with apoE−/− and LDR receptor−/− mice again showed how β3 deficiency was associated with more atherosclerosis, whereas a high frequency of fatal pneumonitis was also noted.92 Expression of inflammatory markers, such as CD36, CD40L, and CD40 (all known to interact with β3), was increased in the lung and the vessel wall and linked to the loss of a tonic suppressive effect of αvβ3 on diet-induced inflammatory events.
Accelerated re-epithelialization is a feature of β3-deficient mice as a result of an increase in TGF-β1 and enhanced migration of dermal fibroblasts into wounds.93 It was suggested that αvβ3 can suppress TGF-β1-mediated signaling and control the rate of wound healing. However, defective bone resorption was confirmed in β3−/− mice. Dysfunctional osteoclasts resulted in age-dependent osteosclerosis, a finding also reproduced in mice genetically engineered to express the β3 variant Ser752Pro mutation.94,95 Injection of B16 melanoma cells into β3−/− mice led to osteolytic bone metastasis in 74% of wild-type mice but only in 4% of β3−/− mice. It was concluded that αIIbβ3 has a role in tumor cell entry into bone and that αvβ3 in osteoclasts is essential for bone resorption.96 These studies confirm much earlier work from Karpatkin et al who showed that the monoclonal antibody 10E5 reactive with αIIbβ3 afforded protection against metastases in a mouse model reconstituted with human platelets.97
Morgan et al, using Cre/loxP technology in mice, showed that conditional depletion of myeloid β3-integrins (including from osteoclasts), but not from platelets, resulted in osteopetrosis (with significant increases in trabecular bone volume) but no bleeding.8 Tumor growth in these mice was also increased and accompanied by decreased macrophage infiltration confirming an earlier study.8,98 This strongly underlines how β3-integrins have cell-specific roles. Interestingly, osteopetrosis appears more of a problem in LAD-3 disease, a finding linked to the role for kindlin-3 in activation of 3 integrin classes (β1, β2, and β3) expressed on osteoclasts and abrogating the formation of podosomes and sealing zones required for bone resorption.99
Remarkably, β3 knockout mice also show abnormal behavior patterns, suggesting that β3 is involved in brain changes relevant to autism spectrum disorder.100
Relatively few studies have been performed on αIIb-deficient mice, in the first model restricted to blood cells, Tronik-Le Roux et al, while confirming the thrombasthenic phenotype, also noted that Itga2b is expressed in murine hematopoietic progenitors.101 Subsequent studies showed that inactivation of Itga2b led to increased liver and bone marrow progenitor numbers and led to the unexpected finding that αIIbβ3 was expressed on mast cells and that it may influence their differentiation and homing, a finding not yet extrapolated to human disease.102
In conclusion, GT through the numbers of patients affected and the distinct bleeding phenotype is the principle-inherited disorder of an integrin. In contrast to the results obtained for knockout mice, the absence or nonfunctioning of αvβ3 in endothelium, bone, and other tissues, including placenta, has yet to be shown to create a distinct phenotype. The gene encoding αv (ITGAV) is found at chromosome 2q32; remarkably, no mutations have been reported to give inherited disease. Although this may suggest that αv is embryonically lethal, the absence of β3 is not, and so the alternative explanation is that the role of αvβ3 can be taken over by other integrins. In addition, ITGB3 missense mutations may affect the expression and/or function of αIIbβ3 and αvβ3 differently (see “Integrin αvβ3 in thrombasthenia”), giving a different situation to that in most transgenic mouse models where mice totally lack expression of the β3 gene. Yet the results for β3-deficient mice clearly emphasize that postmenopausal bone thickening and the susceptibility of GT patients to have cancer and cardiovascular disease need urgent clarification. GT patients are certainly not protected against venous thrombosis, which might be in line with recent studies in mouse models pointing to a role for the GPIb/VWF axis in DVT.103 The incidence of inflammation in GT patients is also unknown. Studies on large ethnic groups with a predominating mutation may help define phenotypic heterogeneity. Importantly, genotyping within epidemiologic studies is progressing fast, and it is to be hoped that an international collaboration with the inclusion of 400 to 500 genotyped patients will soon be possible. This will allow the separation of distinct cohorts and the application of a standardized bleeding score as well as provide a more complete comparison of the different parameters emphasized in this review.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by Inserm (ANR-08-GENO-028-03) and GIS Maladies Rares.
An updated database of mutations giving rise to GT is available at http://sinaicentral.mssm.edu/intranet/research/glanzmann.
Authorship
Contribution: A.T.N. and P.N. designed and wrote the manuscript; M.F. and X.P. collected data and designed figures; X.P. performed the computer modeling; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan T. Nurden, Centre de Référence des Pathologies Plaguettaires, Plateforme Technologique et d' Innovation Biomédicale, Hôpital Xavier Arnozan, 33600 Pessac, France; e-mail: alan.nurden@cnrshl.u-bordeaux2.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal