Abstract
The primary objective of this 2-part phase 1/2 study was to determine the maximum-tolerated dose (MTD) of the potent and selective Aurora B kinase inhibitor barasertib (AZD1152) in patients with newly diagnosed or relapsed acute myeloid leukemia (AML). Part A determined the MTD of barasertib administered as a continuous 7-day infusion every 21 days. In part B, the efficacy of barasertib was evaluated at the MTD. In part A, 32 patients were treated with barasertib 50 mg (n = 3), 100 mg (n = 3), 200 mg (n = 3), 400 mg (n = 4), 800 mg (n = 7), 1200 mg (n = 6), and 1600 mg (n = 6). Dose-limiting toxicities (stomatitis/mucosal inflammation events) were reported in the 800 mg (n = 1), 1200 mg (n = 1), and 1600 mg (n = 2) groups. The MTD was defined as 1200 mg. In part B, 32 patients received barasertib 1200 mg. In each part of the study, 8 of 32 patients had a hematologic response according to Cheson AML criteria. The most commonly reported grade ≥ 3 events were febrile neutropenia (n = 24) and stomatitis/mucosal inflammation (n = 16). We concluded that the MTD of barasertib is 1200 mg in patients with relapsed or newly diagnosed AML. Toxicity was manageable and barasertib treatment resulted in an overall hematologic response rate of 25%. This study is registered at www.ClinicalTrials.gov as NCT00497991.
Introduction
Acute myeloid leukemia (AML) is characterized by an increased number of myeloid cells in the BM and an arrest in their maturation.1 The incidence of AML increases exponentially with age, with most AML patients being ≥ 60 years of age.1,2 The overall 5-year survival is only 40% for patients < 65 years of age (with many relapsing with resistant disease), but this decreases to 10% in patients ≥ 65 years of age; the median survival time is generally ≤ 1 year for older patients receiving standard treatment.1,3,4 Furthermore, the older patient population is less able to tolerate intensive chemotherapy regimens, has a higher incidence of poor-risk cytogenetic abnormalities, and is more likely to express multidrug resistance genes, which reduces the effectiveness of chemotherapy.2,5 Therefore, there is a continuing need to develop more effective treatments for AML patients.
Aurora kinases are a family of proteins (Aurora A, B, and C) known to play an important role in the regulation of mitosis and chromosomal segregation.6 Aurora B kinase is involved in the spindle assembly check-point component of the mitotic process and is overexpressed in a variety of cancers.7 Aurora A kinase is commonly amplified in solid tumors, and the Aurora A gene has been established as an oncogene.8 Aurora C kinase has similar structural and localization properties to Aurora B kinase and is implicated in mammalian spermatogenesis.8 Aurora A kinase has historically been the one most closely associated with tumorigenesis; however, several studies have highlighted a role for Aurora B kinase in oncogenic transformation.9,10
Barasertib (AZD1152) is a selective inhibitor of Aurora B kinase that can inhibit the growth of tumor cells, including those of AML origin.11-15 Furthermore, barasertib has been shown to significantly inhibit the growth of human colon, lung, and hematologic tumor xenografts.12,16 Barasertib was generally well tolerated in a phase 1 study in patients with advanced solid tumors, with neutropenia being the most frequently reported adverse event (AE).17 In clinical studies of other Aurora kinase inhibitors, the most frequently reported AEs were myelotoxicity (particularly febrile neutropenia), stomatitis/mucosal inflammation events, and alopecia.18,19
This phase 1/2 study was conducted to determine the maximum-tolerated dose (MTD) of barasertib and to evaluate the safety, efficacy, and pharmacokinetics (PK) of barasertib in patients with newly diagnosed or relapsed AML.
Methods
Patient selection
Eligibility criteria included patients aged ≥ 18 years with advanced AML for whom no standard therapy existed or no standard therapies were anticipated to result in durable remission (patients in first relapse must have relapsed ≥ 1 month after their initial response) or patients with newly diagnosed AML who were not considered to be suitable for standard induction and consolidation chemotherapy for medical, social, or psychological reasons. A minimum of 15 patients in first relapse were to be recruited to part B of the study. Patients were also required to have a World Health Organization (WHO) performance status of 0-2 and not to have received myeloablative therapy with allogeneic BM or stem cell transplantation within the previous year nor any anticancer agent within 2 weeks before the first dose of study drug. Hydroxyurea was permitted up to 3 days before the first dose of study drug. Exclusion criteria included: serum creatinine ≥ 1.5 × the upper limit of normal (ULN); 24-hour creatinine clearance ≤ 50 mL/min; serum bilirubin > 1.5 × ULN (unless considered due to leukemic organ involvement; Gilbert or a related syndrome was allowed); aspartate aminotransferase or alanine aminotransferase > 2.5 × ULN; previous myeloablative therapy; administration of anticancer agents within 2 weeks before the start of study treatment; French-American-British M3 classification (acute promyelocytic leukemia) of AML; or mean QTc ≥ 470 ms.
All patients provided written informed consent. The study was approved by the independent ethics committee for each trial center and was conducted in accordance with the Declaration of Helsinki.
Study design
This was a phase 1/2, multicenter, open-label, 2-part study designed to assess the safety, efficacy, and PK of barasertib. In part A (phase 1), barasertib was administered as a continuous 7-day infusion at doses of 50, 100, 200, 400, 800, 1200, and 1600 mg every 21 days until the patient was withdrawn because of toxicity or, in the opinion of the investigator, was no longer receiving benefit from the treatment. It was anticipated that patients (in both parts A and B) would receive 3 cycles of treatment, although they could continue with treatment if, in the opinion of the investigator, they continued to benefit. Cohorts of ≥ 3 patients received escalating doses of barasertib until the nontolerated dose was reached, defined as the dose at which a minimum of 2 patients experienced a dose-limiting toxicity (DLT). In the absence of a DLT, dose escalation to the next dose level occurred once 3 evaluable patients had been followed for 21 days. If 1 DLT occurred, that dose cohort was expanded to a maximum of 6 patients. The MTD was the dose level at which none or 1 patient had a DLT, with at least 2 patients with a DLT at the next higher dose level. In part B (phase 2), barasertib was administered at the MTD established in part A according to the same treatment regimen.
The primary objectives of the study in part A (dose escalation) were to establish the MTD and to assess the safety and tolerability of multiple ascending doses of barasertib. Secondary objectives included assessments of efficacy and PK. The primary objective in part B (dose expansion) was to assess the efficacy of barasertib at the MTD established in part A; secondary objectives included assessments of safety, tolerability, and PK.
Toxicity criteria
The incidence and severity of AEs were evaluated throughout the study according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. A DLT was defined as a grade 3, 4, or 5 nonhematologic (including biochemical) toxicity despite adequate supportive care and considered related to barasertib by the study investigator.
Response evaluation
Response evaluations were performed every 21 days based on BM and blood assessments. Complete remission (CR), complete remission with incomplete recovery of neutrophils or platelets (CRi), and partial remission (PR) were evaluated based on the International Working Group AML clinical response criteria.20 Duration of response was calculated as the time from documentation of remission to the earliest date of relapse (as defined by the reappearance of leukemic blasts in the blood or > 5% leukemic blasts in the BM, recurrence of a previously documented cytogenetic or molecular abnormality, the reappearance of new dysplastic changes, or the reappearance or development of extramedullary leukemia). BM samples (3 mL) were collected predose in cycle 1 for cytogenetic assessment of Medical Research Council (MRC) prognostic group (favorable, intermediate, or adverse) in patients for whom the required cytogenetic information from a routine BM sample was not already collected within 3 months before the study.
PK
Barasertib is a prodrug metabolized in plasma to its more active form, barasertib-hQPA. In this study, plasma concentrations of both barasertib and barasertib-hQPA were determined during and after 7-day infusions of barasertib. In part A, blood samples were taken at the following times in cycle 1: predose; 24, 48, and 120 hours after the start of infusion (SOI); 5 minutes before the end of infusion (EOI); 0.25, 0.5, 0.75, 1, 2, 4, 6, and 24 hours after the EOI; and on days 15 and 18. During the subsequent 2 cycles, blood samples were taken predose, 120 hours after the SOI; 5 minutes before the EOI; 1, 2, 6, and 24 hours after the EOI, and on day 15.
In part B, blood samples were taken at the following times in cycle 1: predose; 24, 48, and 120 hours after SOI and 5 minutes before the EOI; and 0.25, 1, 2, 6, and 24 hours after the EOI and on day 15. During cycle 2, blood samples were taken predose; 120 hours after the SOI; 5 minutes before the EOI on day 8; 6 and 24 hours after the EOI; and on day 15. The PK parameters assessed included: steady-state plasma concentration (Css); area under the concentration-time curve (AUC) from 0 to infinity; area under the concentration-time curve from 0 to 192 hours (AUC0-192h); elimination half-life (t1/2); clearance (CL); volume of distribution (Vss); and volume of distribution (apparent) during the terminal (λz) phase (Vz).
Plasma PK parameters of barasertib and barasertib-hQPA were determined by noncompartmental methods, using WinNonlin Enterprise Version 5.2.1 software (Pharsight).
Statistical analysis
Part A of the study was designed so that at least 6 evaluable patients were recruited at the dose deemed to be the MTD. No formal sample size calculations were undertaken for part B; however, 30 evaluable patients were considered sufficient (of whom 15 patients were to be in first relapse) to make a preliminary assessment of the efficacy of barasertib in AML. An 80% confidence interval (80% CI) was provided for the response rates based on the Wilson score method.21
Results
Between May 2006 and October 2009, 64 patients received barasertib (32 patients in each part of the study). Baseline patient demographics and characteristics are summarized in Table 1.
Baseline patient demographics and characteristics, and barasertib treatment cycles
| . | Barasertib dose . | |||||||
|---|---|---|---|---|---|---|---|---|
| Part A . | Part B . | |||||||
| 50 mg (n = 3) . | 100 mg (n = 3) . | 200 mg (n = 3) . | 400 mg (n = 4) . | 800 mg (n = 7) . | 1200 mg (n = 6) . | 1600 mg (n = 6) . | 1200 mg (n = 32) . | |
| Median age, y (range) | 66.0 (46-75) | 73.0 (65-82) | 60.0 (56-62) | 67.5 (66-80) | 64.0 (59-74) | 68.0 (45-75) | 65.0 (57-76) | 69.0 (48-87) |
| Male sex, n (%) | 2 (67) | 2 (67) | 1 (33) | 3 (75) | 5 (71) | 3 (50) | 2 (33) | 19 (59) |
| WHO performance status, n (%) | ||||||||
| 0 | 0 | 3 (100) | 1 (33) | 3 (75) | 4 (57) | 2 (33) | 3 (50) | 16 (50) |
| 1 | 2 (67) | 0 | 1 (33) | 1 (25) | 2 (29) | 3 (50) | 1 (17) | 13 (41) |
| 2 | 1 (33) | 0 | 1 (33) | 0 | 1 (14) | 1 (17) | 2 (33) | 3 (9) |
| AML type, n (%) | ||||||||
| De novo | 1 (33) | 2 (67) | 1 (33) | 0 | 6 (86) | 2 (33) | 3 (50) | 10 (31) |
| Secondary to myelodysplastic syndrome or myeloproliferative disorder | 2 (67) | 1 (33) | 2 (67) | 3 (75) | 0 | 2 (33) | 3 (50) | 12 (37) |
| Secondary to chemotherapy | 0 | 0 | 0 | 1 (25) | 1 (14) | 1 (17) | 0 | 4 (13) |
| Other* | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 6 (19) |
| AML status, n (%) | ||||||||
| Newly diagnosed | 0 | 0 | 0 | 1 (25) | 0 | 2 (33) | 1 (17) | 15 (47) |
| First relapse | 3 (100) | 3 (100) | 3 (100) | 2 (50) | 6 (86) | 4 (67) | 4 (67) | 12 (38) |
| Second relapse | 0 | 0 | 0 | 1 (25) | 0 | 0 | 0 | 3 (9) |
| Missing | 0 | 0 | 0 | 0 | 1 (14) | 0 | 1 (17) | 2 (6) |
| Time to relapse for patients in first relapse, n (%)† | ||||||||
| <6 months | 1 (33) | 0 | 1 (33) | 0 | 0 | 1 (17) | 0 | 1 (3) |
| 6 to < 12 months | 2 (67) | 1 (33) | 1 (33) | 0 | 0 | 0 | 0 | 2 (6) |
| ≥12 months | 0 | 1 (33) | 0 | 2 (50) | 0 | 0 | 1 (17) | 0 |
| Missing | 0 | 1 (33) | 1 (33) | 0 | 6 (86) | 3 (50) | 3 (50) | 9 (28) |
| Cytogenetic information, n (%) | ||||||||
| Normal | 2 (67) | 0 | 2 (67) | 1 (25) | 2 (29) | 0 | 1 (17) | 8 (25) |
| Complex (>3 abnormalities) | 1 (33) | 0 | 0 | 0 | 0 | 4 (67) | 0 | 1 (3) |
| Abnormalities of 5 and/or 7 | 0 | 0 | 1 (33) | 1 (25) | 0 | 1 (17) | 1 (17) | 3 (9) |
| Other | 0 | 1 (33) | 0 | 1 (25) | 3 (43) | 0 | 2 (33) | 9 (28) |
| Not available | 0 | 2 (67) | 0 | 1 (25) | 2 (29) | 1 (17) | 2 (33) | 11 (34) |
| Number of prior chemotherapy regimens, n (%) | ||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 2 (6) |
| 1 | 0 | 1 (33) | 0 | 0 | 1 (14) | 0 | 2 (33) | 5 (16) |
| 2 | 0 | 0 | 0 | 2 (50) | 3 (43) | 1 (17) | 1 (17) | 13 (41) |
| ≥3 | 3 (100) | 2 (67) | 3 (100) | 2 (50) | 3 (43) | 4 (67) | 3 (50) | 12 (38) |
| Prior radiotherapy, n (%) | ||||||||
| No | 3 (100) | 3 (100) | 3 (100) | 3 (75) | 7 (100) | 6 (100) | 5 (83) | 29 (91) |
| Yes | 0 | 0 | 0 | 1 (25) | 0 | 0 | 1 (17) | 3 (9) |
| Number of cycles of barasertib received, n (%) | ||||||||
| ≥1 | 3 (100) | 3 (100) | 3 (100) | 4 (100) | 7 (100) | 6 (100) | 6 (100) | 32 (100) |
| ≥2 | 2 (67) | 3 (100) | 1 (33) | 3 (75) | 5 (71) | 2 (33) | 3 (50) | 16 (50) |
| ≥3 | 1 (33) | 2 (67) | 0 | 1 (25) | 3 (43) | 2 (33) | 1 (17) | 8 (25) |
| ≥4 | 0 | 0 | 0 | 0 | 1 (14) | 0 | 0 | 2 (6) |
| ≥5 | 0 | 0 | 0 | 0 | 1 (14) | 0 | 0 | 1 (3) |
| ≥6 | 0 | 0 | 0 | 0 | 1 (14) | 0 | 0 | 1 (3) |
| . | Barasertib dose . | |||||||
|---|---|---|---|---|---|---|---|---|
| Part A . | Part B . | |||||||
| 50 mg (n = 3) . | 100 mg (n = 3) . | 200 mg (n = 3) . | 400 mg (n = 4) . | 800 mg (n = 7) . | 1200 mg (n = 6) . | 1600 mg (n = 6) . | 1200 mg (n = 32) . | |
| Median age, y (range) | 66.0 (46-75) | 73.0 (65-82) | 60.0 (56-62) | 67.5 (66-80) | 64.0 (59-74) | 68.0 (45-75) | 65.0 (57-76) | 69.0 (48-87) |
| Male sex, n (%) | 2 (67) | 2 (67) | 1 (33) | 3 (75) | 5 (71) | 3 (50) | 2 (33) | 19 (59) |
| WHO performance status, n (%) | ||||||||
| 0 | 0 | 3 (100) | 1 (33) | 3 (75) | 4 (57) | 2 (33) | 3 (50) | 16 (50) |
| 1 | 2 (67) | 0 | 1 (33) | 1 (25) | 2 (29) | 3 (50) | 1 (17) | 13 (41) |
| 2 | 1 (33) | 0 | 1 (33) | 0 | 1 (14) | 1 (17) | 2 (33) | 3 (9) |
| AML type, n (%) | ||||||||
| De novo | 1 (33) | 2 (67) | 1 (33) | 0 | 6 (86) | 2 (33) | 3 (50) | 10 (31) |
| Secondary to myelodysplastic syndrome or myeloproliferative disorder | 2 (67) | 1 (33) | 2 (67) | 3 (75) | 0 | 2 (33) | 3 (50) | 12 (37) |
| Secondary to chemotherapy | 0 | 0 | 0 | 1 (25) | 1 (14) | 1 (17) | 0 | 4 (13) |
| Other* | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 6 (19) |
| AML status, n (%) | ||||||||
| Newly diagnosed | 0 | 0 | 0 | 1 (25) | 0 | 2 (33) | 1 (17) | 15 (47) |
| First relapse | 3 (100) | 3 (100) | 3 (100) | 2 (50) | 6 (86) | 4 (67) | 4 (67) | 12 (38) |
| Second relapse | 0 | 0 | 0 | 1 (25) | 0 | 0 | 0 | 3 (9) |
| Missing | 0 | 0 | 0 | 0 | 1 (14) | 0 | 1 (17) | 2 (6) |
| Time to relapse for patients in first relapse, n (%)† | ||||||||
| <6 months | 1 (33) | 0 | 1 (33) | 0 | 0 | 1 (17) | 0 | 1 (3) |
| 6 to < 12 months | 2 (67) | 1 (33) | 1 (33) | 0 | 0 | 0 | 0 | 2 (6) |
| ≥12 months | 0 | 1 (33) | 0 | 2 (50) | 0 | 0 | 1 (17) | 0 |
| Missing | 0 | 1 (33) | 1 (33) | 0 | 6 (86) | 3 (50) | 3 (50) | 9 (28) |
| Cytogenetic information, n (%) | ||||||||
| Normal | 2 (67) | 0 | 2 (67) | 1 (25) | 2 (29) | 0 | 1 (17) | 8 (25) |
| Complex (>3 abnormalities) | 1 (33) | 0 | 0 | 0 | 0 | 4 (67) | 0 | 1 (3) |
| Abnormalities of 5 and/or 7 | 0 | 0 | 1 (33) | 1 (25) | 0 | 1 (17) | 1 (17) | 3 (9) |
| Other | 0 | 1 (33) | 0 | 1 (25) | 3 (43) | 0 | 2 (33) | 9 (28) |
| Not available | 0 | 2 (67) | 0 | 1 (25) | 2 (29) | 1 (17) | 2 (33) | 11 (34) |
| Number of prior chemotherapy regimens, n (%) | ||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 2 (6) |
| 1 | 0 | 1 (33) | 0 | 0 | 1 (14) | 0 | 2 (33) | 5 (16) |
| 2 | 0 | 0 | 0 | 2 (50) | 3 (43) | 1 (17) | 1 (17) | 13 (41) |
| ≥3 | 3 (100) | 2 (67) | 3 (100) | 2 (50) | 3 (43) | 4 (67) | 3 (50) | 12 (38) |
| Prior radiotherapy, n (%) | ||||||||
| No | 3 (100) | 3 (100) | 3 (100) | 3 (75) | 7 (100) | 6 (100) | 5 (83) | 29 (91) |
| Yes | 0 | 0 | 0 | 1 (25) | 0 | 0 | 1 (17) | 3 (9) |
| Number of cycles of barasertib received, n (%) | ||||||||
| ≥1 | 3 (100) | 3 (100) | 3 (100) | 4 (100) | 7 (100) | 6 (100) | 6 (100) | 32 (100) |
| ≥2 | 2 (67) | 3 (100) | 1 (33) | 3 (75) | 5 (71) | 2 (33) | 3 (50) | 16 (50) |
| ≥3 | 1 (33) | 2 (67) | 0 | 1 (25) | 3 (43) | 2 (33) | 1 (17) | 8 (25) |
| ≥4 | 0 | 0 | 0 | 0 | 1 (14) | 0 | 0 | 2 (6) |
| ≥5 | 0 | 0 | 0 | 0 | 1 (14) | 0 | 0 | 1 (3) |
| ≥6 | 0 | 0 | 0 | 0 | 1 (14) | 0 | 0 | 1 (3) |
In part A, other AML type was newly diagnosed AML classified as secondary to chemotherapy, myelodysplastic syndrome, and chronic lymphocytic leukemia for 1 patient in part A; in part B, other AML type includes 4 newly diagnosed patients (1 in first relapse, 2 with chronic myelomonocytic leukemia, and 1 with newly diagnosed AML), 1 patient in first relapse, and 1 patient in second relapse.
Time to first relapse after a CR and before treatment with barasertib.
Patients, treatment, and dose-limiting toxicities in part A
In part A, 32 patients were treated with barasertib at dose levels of 50 mg (n = 3), 100 mg (n = 3), 200 mg (n = 3), 400 mg (n = 4), 800 mg (n = 7), 1200 mg (n = 6), and 1600 mg (n = 6). All patients received ≥ 1 cycle of treatment (Table 1). Of the 19 patients who received ≥ 2 treatment cycles, 13 patients had a dose delay of ≥ 1 week. At the time of data cutoff (October 14, 2009), all patients had discontinued treatment because of lack of therapeutic response (17 of 32; 53%), worsening of clinical condition (4 of 32; 13%), AEs (4 of 32; 13%), death due to disease progression (5 of 32; 16%), or voluntary discontinuation (2 of 32; 6%). Four DLTs (all stomatitis/mucosal inflammation events) were reported in the barasertib 800 mg (n = 1), 1200 mg (n = 1), and 1600 mg (n = 2) groups. The MTD of barasertib was defined as 1200 mg. No dose-related renal or hepatic effects were observed, as assessed by changes in creatinine clearance and aspartate aminotransferase, alanine aminotransferase, and bilirubin levels.
Patients and treatment in part B
In part B, 32 patients received barasertib 1200 mg. All 32 patients received ≥ 1 cycle of treatment, 16 received ≥ 2 cycles, and 1 had received 6 cycles to date (Table 1). Of the 16 patients who received ≥ 2 treatment cycles, 13 patients had a dose delay of ≥ 1 week. At the time of data cutoff, 31 (97%) patients had discontinued treatment; 18 (56%) discontinued because of lack of response, 6 (19%) because of worsening clinical condition, 3 (9%) died, and 4 (13%) voluntarily discontinued (1 had a clinical response of CR and 2 had a CRi before they discontinued).
Efficacy
Eight (25%) patients each in part A and part B had hematologic responses (CR/CRi/PR; Table 2). The overall response rate (CR/CRi/PR) was 25% (80% CI, 19%-33%) for study parts A and B combined (n = 64) and 26% (80% CI, 18%-36%) for patients who received the MTD of 1200 mg of barasertib in both parts combined (n = 38). The CR/CRi response rate was 14% (80% CI, 9%-21%) for study parts A and B combined and 18% (80% CI, 12%-28%) for patients who received 1200 mg in both parts combined. The durations of CR were 23, 58, and 115 days, and for CRi were 7, 23, 25, 27, 29, and 206 days. The duration of response was censored at 23, 27, and 115 days for 3 patients who voluntarily discontinued from the study while still in remission, and at 206 days for 1 patient who was still in remission at data cutoff. Responses were seen across all MRC prognostic cytogenetic groups,22 the adverse (n = 4), intermediate (n = 8), and favorable (n = 1) categories (cytogenetic assessments were not available for 3 of the responding patients). Responses were also seen across all AML status groups; for patients who received barasertib 1200 mg, response rates were 17.6% for newly diagnosed patients, 37.5% for those in first relapse, and 33.3% for those in second relapse.
Hematological responses in patients in parts A and B
| Response . | Barasertib dose . | |||||||
|---|---|---|---|---|---|---|---|---|
| Part A . | Part B . | |||||||
| 50 mg (n = 3) . | 100 mg (n = 3) . | 200 mg (n = 3) . | 400 mg (n = 4) . | 800 mg (n = 7) . | 1200 mg (n = 6) . | 1600 mg (n = 6) . | 1200 mg (n = 32) . | |
| CR, n (%) | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 2 (6) |
| Cri, n (%) | 0 | 0 | 0 | 1 (25) | 1 (14) | 0 | 0 | 4 (13)* |
| PR, n (%) | 0 | 1 (33) | 0 | 0 | 1 (14) | 1 (17) | 2 (33) | 2 (6) |
| Response . | Barasertib dose . | |||||||
|---|---|---|---|---|---|---|---|---|
| Part A . | Part B . | |||||||
| 50 mg (n = 3) . | 100 mg (n = 3) . | 200 mg (n = 3) . | 400 mg (n = 4) . | 800 mg (n = 7) . | 1200 mg (n = 6) . | 1600 mg (n = 6) . | 1200 mg (n = 32) . | |
| CR, n (%) | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 2 (6) |
| Cri, n (%) | 0 | 0 | 0 | 1 (25) | 1 (14) | 0 | 0 | 4 (13)* |
| PR, n (%) | 0 | 1 (33) | 0 | 0 | 1 (14) | 1 (17) | 2 (33) | 2 (6) |
One patient in Part B was not transfusion independent at the time of CRi.
Safety and tolerability
In part A, all 32 patients had at least one AE, the majority of which were grade 1/2 in severity. The most commonly reported grade 3/4 events were febrile neutropenia and stomatitis/mucosal inflammation events (Table 3). Of the 13 patients with febrile neutropenia; 11 had grade 3/4 febrile neutropenia. Twenty-eight stomatitis/mucosal inflammation events were reported; 7 patients had 11 grade 3/4 events. Two patients receiving barasertib 1200 mg were hospitalized for grade 3 stomatitis; 1 for 9 days and 1 for an unknown duration (the patient was withdrawn from the study because of disease progression). One patient receiving barasertib 1600 mg was hospitalized for grade 3 stomatitis (concurrently with an event of febrile neutropenia) for a total of 18 days. AEs leading to dose delay were experienced by 6 patients and were catheter-related complications (1600 mg), stomatitis/mucosal inflammation events (1600 mg), pseudomonal sepsis and cellulitis (1200 mg), febrile BM aplasia (800 mg), cholecystitis acute (400 mg), and local swelling (400 mg). All events resolved after dose delay apart from the febrile BM aplasia (ongoing at data cutoff). There were 8 (25%) deaths, all related to disease progression.
AEs reported by ≥ 20% of patients overall (in either part A or B) split by maximum grade achieved
| . | Patients with AEs, n (%) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Part A barasertib dose . | Part B barasertib dose . | ||||||||
| 50 mg (n = 3) . | 100 mg (n = 3) . | 200 mg (n = 3) . | 400 mg (n = 4) . | 800 mg (n = 7) . | 1200 mg (n = 6) . | 1600 mg (n = 6) . | Total (n = 32) . | 1200 mg (n = 32) . | |
| Any event | 3 (100) | 3 (100) | 3 (100) | 4 (100) | 7 (100) | 6 (100) | 6 (100) | 32 (100) | 32 (100) |
| Grade ≥ 3 | 3 (100) | 1 (33) | 3 (100) | 4 (100) | 7 (100) | 5 (83) | 5 (83) | 28 (88) | 24 (75) |
| Febrile neutropenia* | 13 (41) | 15 (47) | |||||||
| Grade 1/2 | 0 | 0 | 1 (33) | 0 | 0 | 0 | 1 (17) | 2 (6) | 2 (6) |
| Grade ≥ 3 | 0 | 0 | 0 | 4 (100) | 2 (29) | 2 (33) | 3 (50) | 11 (34) | 13 (41) |
| Stomatitis/mucosal inflammation events | 12 (38) | 22 (69) | |||||||
| Grade 1/2 | 0 | 0 | 1 (33) | 0 | 2 (28) | 2 (33) | 0 | 5 (16) | 13 (41) |
| Grade ≥ 3 | 0 | 0 | 0 | 1 (25) | 1 (14) | 2 (33) | 3 (50) | 7 (22) | 9 (28) |
| Pyrexia | 13 (41) | 14 (44) | |||||||
| Grade 1/2 | 1 (33) | 0 | 2 (67) | 1 (25) | 3 (43) | 4 (67) | 0 | 11 (34) | 12 (38) |
| Grade ≥ 3 | 0 | 1 (33) | 0 | 0 | 1 (14) | 0 | 0 | 2 (6) | 2 (6) |
| Nausea | 13 (41) | 12 (38) | |||||||
| Grade 1/2 | 0 | 1 (33) | 1 (33) | 2 (50) | 3 (43) | 3 (50) | 3 (50) | 13 (41) | 11 (34) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3) |
| Fatigue | 10 (31) | 15 (47) | |||||||
| Grade 1/2 | 0 | 1 (33) | 0 | 1 (25) | 2 (29) | 4 (67) | 2 (33) | 10 (31) | 12 (38) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (9) |
| Diarrhea | 9 (28) | 17 (53) | |||||||
| Grade 1/2 | 0 | 0 | 1 (33) | 0 | 1 (14) | 4 (67) | 2 (33) | 9 (28) | 17 (53) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipation | 11 (34) | 5 (15) | |||||||
| Grade 1/2 | 0 | 1 (33) | 1 (33) | 1 (25) | 2 (29) | 4 (67) | 1 (17) | 10 (31) | 5 (15) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 1 (14) | 0 | 0 | 1 (3) | 0 |
| Vomiting | 8 (25) | 9 (28) | |||||||
| Grade 1/2 | 1 (33) | 0 | 0 | 0 | 3 (43) | 2 (33) | 2 (33) | 8 (25) | 9 (28) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Peripheral edema | 9 (28) | 7 (22) | |||||||
| Grade 1/2 | 2 (67) | 1 (33) | 0 | 1 (25) | 1 (14) | 1 (17) | 2 (33) | 8 (25) | 6 (19) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (3) | 1 (3) |
| Headache | 4 (13) | 9 (28) | |||||||
| Grade 1/2 | 1 (33) | 1 (33) | 0 | 0 | 0 | 1 (17) | 0 | 3 (9) | 9 (28) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) | 1 (3) | 0 |
| Alopecia | 5 (16) | 8 (25) | |||||||
| Grade 1/2 | 0 | 0 | 0 | 1 (25) | 3 (43) | 0 | 1 (17) | 5 (16) | 8 (25) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Epistaxis | 5 (16) | 8 (25) | |||||||
| Grade 1/2 | 0 | 0 | 0 | 1 (33) | 1 (14) | 1 (17) | 1 (17) | 4 (13) | 7 (22) |
| Grade ≥ 3 | 1 (33) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3) | 1 (3) |
| . | Patients with AEs, n (%) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Part A barasertib dose . | Part B barasertib dose . | ||||||||
| 50 mg (n = 3) . | 100 mg (n = 3) . | 200 mg (n = 3) . | 400 mg (n = 4) . | 800 mg (n = 7) . | 1200 mg (n = 6) . | 1600 mg (n = 6) . | Total (n = 32) . | 1200 mg (n = 32) . | |
| Any event | 3 (100) | 3 (100) | 3 (100) | 4 (100) | 7 (100) | 6 (100) | 6 (100) | 32 (100) | 32 (100) |
| Grade ≥ 3 | 3 (100) | 1 (33) | 3 (100) | 4 (100) | 7 (100) | 5 (83) | 5 (83) | 28 (88) | 24 (75) |
| Febrile neutropenia* | 13 (41) | 15 (47) | |||||||
| Grade 1/2 | 0 | 0 | 1 (33) | 0 | 0 | 0 | 1 (17) | 2 (6) | 2 (6) |
| Grade ≥ 3 | 0 | 0 | 0 | 4 (100) | 2 (29) | 2 (33) | 3 (50) | 11 (34) | 13 (41) |
| Stomatitis/mucosal inflammation events | 12 (38) | 22 (69) | |||||||
| Grade 1/2 | 0 | 0 | 1 (33) | 0 | 2 (28) | 2 (33) | 0 | 5 (16) | 13 (41) |
| Grade ≥ 3 | 0 | 0 | 0 | 1 (25) | 1 (14) | 2 (33) | 3 (50) | 7 (22) | 9 (28) |
| Pyrexia | 13 (41) | 14 (44) | |||||||
| Grade 1/2 | 1 (33) | 0 | 2 (67) | 1 (25) | 3 (43) | 4 (67) | 0 | 11 (34) | 12 (38) |
| Grade ≥ 3 | 0 | 1 (33) | 0 | 0 | 1 (14) | 0 | 0 | 2 (6) | 2 (6) |
| Nausea | 13 (41) | 12 (38) | |||||||
| Grade 1/2 | 0 | 1 (33) | 1 (33) | 2 (50) | 3 (43) | 3 (50) | 3 (50) | 13 (41) | 11 (34) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3) |
| Fatigue | 10 (31) | 15 (47) | |||||||
| Grade 1/2 | 0 | 1 (33) | 0 | 1 (25) | 2 (29) | 4 (67) | 2 (33) | 10 (31) | 12 (38) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (9) |
| Diarrhea | 9 (28) | 17 (53) | |||||||
| Grade 1/2 | 0 | 0 | 1 (33) | 0 | 1 (14) | 4 (67) | 2 (33) | 9 (28) | 17 (53) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipation | 11 (34) | 5 (15) | |||||||
| Grade 1/2 | 0 | 1 (33) | 1 (33) | 1 (25) | 2 (29) | 4 (67) | 1 (17) | 10 (31) | 5 (15) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 1 (14) | 0 | 0 | 1 (3) | 0 |
| Vomiting | 8 (25) | 9 (28) | |||||||
| Grade 1/2 | 1 (33) | 0 | 0 | 0 | 3 (43) | 2 (33) | 2 (33) | 8 (25) | 9 (28) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Peripheral edema | 9 (28) | 7 (22) | |||||||
| Grade 1/2 | 2 (67) | 1 (33) | 0 | 1 (25) | 1 (14) | 1 (17) | 2 (33) | 8 (25) | 6 (19) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 1 (17) | 0 | 1 (3) | 1 (3) |
| Headache | 4 (13) | 9 (28) | |||||||
| Grade 1/2 | 1 (33) | 1 (33) | 0 | 0 | 0 | 1 (17) | 0 | 3 (9) | 9 (28) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (17) | 1 (3) | 0 |
| Alopecia | 5 (16) | 8 (25) | |||||||
| Grade 1/2 | 0 | 0 | 0 | 1 (25) | 3 (43) | 0 | 1 (17) | 5 (16) | 8 (25) |
| Grade ≥ 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Epistaxis | 5 (16) | 8 (25) | |||||||
| Grade 1/2 | 0 | 0 | 0 | 1 (33) | 1 (14) | 1 (17) | 1 (17) | 4 (13) | 7 (22) |
| Grade ≥ 3 | 1 (33) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3) | 1 (3) |
Febrile neutropenia was classed as CTCAE Grade ≥ 3, some febrile neutropenia events of Grade 1 and 2 were reported in error by the investigators.
As in part A, all 32 patients in part B experienced at least one AE, the majority of which were grade 1/2; the most commonly reported grade 3/4 events were febrile neutropenia and stomatitis/mucosal inflammation events (Table 3). Twenty-three febrile neutropenia events were experienced in 15 patients; grade 3 events were reported in 13 of these patients (Table 3). Thirteen of the grade 3 events (occurring in 9 patients) were considered serious and 6 of these events were considered to be treatment related. Febrile neutropenia resolved after dose delay in all but one patient. Fifty-four stomatitis/mucosal inflammation events were reported in 22 patients, of whom 9 experienced 11 grade 3 events. Eight patients had grade 1/2 alopecia, which was considered to be treatment related and ongoing at data cutoff. No AEs resulted in treatment discontinuation. There were 5 deaths (16%), 2 because of disease progression alone, 1 because of disease progression and serious AE (Escherichia sepsis), 1 because of a serious AE of cerebral fungal infection; the primary cause of death was not reported for the other patient, but the death was not because of disease progression and was not considered by the investigator to be related to barasertib treatment.
PK
In part A, after a 7-day infusion of barasertib (50-1600 mg), exposure to barasertib-hQPA increased in a dose-proportional manner, with a 31-fold increase in the geometric mean Css or AUC0-192h for the 32-fold increase in dose (data not shown). When exposure was normalized for dose, patient weight did not appear to be significantly correlated to exposure.
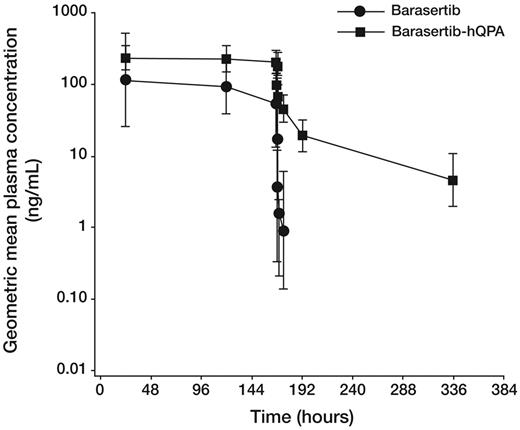
In part B, after a 7-day infusion of barasertib 1200 mg, plasma concentrations of barasertib-hQPA reached a plateau by the time of the first sample, which was taken at 24 hours into the infusion. During the infusion period, barasertib-hQPA exposure was ∼ 3-fold higher compared with barasertib (Figure 1 and Table 4). After the EOI, the plasma concentrations of barasertib declined very rapidly, approaching the limit of quantification of the assay (0.25 ng/mL) within 6 hours after the EOI. Plasma concentrations of barasertib-hQPA declined in a triphasic manner, with low plasma concentrations (∼ 1.0 ng/mL) still detectable at 336 hours (14 days) after the infusion. Approximately 95% of the exposure to barasertib-hQPA was observed by 24 hours after the EOI. There was no accumulation of barasertib-hQPA upon repeat cycle administration.
Geometric mean plasma concentration-time profiles of barasertib and barasertib-hQPA after a 7-day infusion of barasertib 1200 mg. Error bars indicate SD.
Geometric mean plasma concentration-time profiles of barasertib and barasertib-hQPA after a 7-day infusion of barasertib 1200 mg. Error bars indicate SD.
PK parameters of barasertib and barasertib-hQPA following a 7-day infusion of barasertib 1200 mg in part B
| PK parameter . | Geometric mean (CV, %) [n] . | |
|---|---|---|
| Barasertib (n = 32) . | Barasertib-hQPA (n = 32) . | |
| Css, ng/mL | 87.4 (46.2) [13] | 245.2 (22.7) [15] |
| AUC, ng/h/mL | NC | 40 930 (20.7) [12] |
| AUC0–192h, ng/h/mL | 14 030 (46.0) [13]* | 39 300 (22.3) [15] |
| CL, L/h | NC | 29.3 (22.8) [12] |
| Vss, L | NC | 614.2 (42.7) [12] |
| Vz, L | NC | 3278 (44.1) [12] |
| t1/2, h | NC | 77.5 (34.6) [12] |
| PK parameter . | Geometric mean (CV, %) [n] . | |
|---|---|---|
| Barasertib (n = 32) . | Barasertib-hQPA (n = 32) . | |
| Css, ng/mL | 87.4 (46.2) [13] | 245.2 (22.7) [15] |
| AUC, ng/h/mL | NC | 40 930 (20.7) [12] |
| AUC0–192h, ng/h/mL | 14 030 (46.0) [13]* | 39 300 (22.3) [15] |
| CL, L/h | NC | 29.3 (22.8) [12] |
| Vss, L | NC | 614.2 (42.7) [12] |
| Vz, L | NC | 3278 (44.1) [12] |
| t1/2, h | NC | 77.5 (34.6) [12] |
NC indicates not calculable.
AUC0-192h for barasertib.
Discussion
Treatment of refractory, relapsed AML and AML in elderly patients remains a major challenge because many of these patients are unable to tolerate intensive chemotherapy regimens and are more likely to express multidrug resistance genes, reducing the effectiveness of chemotherapy.2,4,5 In the current study of patients with AML (overall median age 67.5 years, range 45-87), the response rate (CR + CRi + PR) after barasertib treatment for both parts A and B combined was 25% (16 of 64 patients; age range, 52-75 years). These findings provide promising preliminary evidence for the efficacy of this treatment in elderly, refractory, or relapsed AML patients.
Cytogenetic data suggested that responses occurred in patients in all prognostic risk groups (favorable, intermediate, and adverse). Furthermore, these findings provide some encouragement to patients with advanced AML, because a significant number of patients were in relapse after several previous chemotherapy regimens (52 patients, 81%, had ≥ 2 prior chemotherapy regimens) and approximately 48% had secondary AML (ie, therapy-related AML or AML after a myelodysplastic or myeloproliferative hematologic condition). Whereas these data should be interpreted with caution because of the small number of patients per group, response rates for patients in first and second relapse were approximately double those observed for newly diagnosed patients. In contrast to the hematologic responses achieved in this study in patients with AML, previous studies of barasertib and other Aurora A and/or B kinase inhibitors in patients with advanced solid tumors have generally only reported stable disease as the best objective response.18,19,23-27
Barasertib administered as a 7-day infusion every 21 days was generally well tolerated and AEs were manageable in patients with AML. During part A of the study (dose escalation), the DLTs were stomatitis/mucosal inflammation events, and barasertib 1200 mg was identified as the MTD. During part B (dose expansion), the most commonly reported AEs were stomatitis/mucosal inflammation events, diarrhea, fatigue, febrile neutropenia, and pyrexia. The majority of AEs experienced were grade 1/2. However, many patients experienced at least one grade 3/4 stomatitis/mucosal inflammation event and/or febrile neutropenia, the majority of which were manageable and resolved after dose delay. These events were anticipated from previous clinical studies of barasertib and other Aurora kinase inhibitors in patients with solid tumors.17,28,29
Recent studies of other agents with activity against Aurora B kinase have also demonstrated preliminary evidence of anticancer activity: AT9283, an inhibitor of Aurora A, JAK2, and Abl30 in patients with imatinib-refractory chronic myeloid leukemia and AML,31 and AS703569, an inhibitor of Aurora A, B, and C in patients with chronic myeloid leukemia, AML, and myelodysplastic syndrome.32 However, because AT9283 and AS703569 are not selective for Aurora B, it remains to be established whether the anticancer activity observed with these agents is because of an effect on Aurora B and/or other target(s). The efficacy observed in the current study is most likely because of inhibition of Aurora B kinase by barasertib. Barasertib-hQPA, the more active form of the prodrug barasertib, is a highly potent and selective inhibitor of Aurora B kinase compared with Aurora A kinase, and has a high specificity compared with a panel of 50 other kinases.33 Studies assessing the combination of barasertib and low-dose cytosine arabinoside in patients ≥ 60 years of age with AML are currently under way; in one phase 1 safety and tolerability study, the combination was found to have an acceptable tolerability profile, providing preliminary evidence for efficacy.34
In part A of the present study, dose-proportional exposure to barasertib-hQPA was observed across the dose range (50-1600 mg), with a 31-fold increase in the geometric mean Css or AUC0-192h for the 32-fold increase in dose. In part B (dosing at the MTD), the plateau concentration of barasertib-hQPA was approximately 3-fold higher than that of barasertib. The volume of distribution (apparent) during Vz was approximately 5-fold higher than the Vss, indicating that barasertib-hQPA is distributed to tissues. However, the rate of distribution was relatively slow, with the large difference between Vz and Vss values resulting from the majority of barasertib-hQPA being eliminated before the distribution equilibrium was attained. Therefore, the time taken to achieve the plateau concentration of barasertib-hQPA during infusion of barasertib is controlled by the distribution kinetics rather than by the long half-life of barasertib-hQPA (geometric mean, 55.8 hours). Similar PK findings have been reported in previous phase 1 studies of barasertib administered by various infusion schedules in patients with solid tumors (AstraZeneca data on file).17
In summary, the MTD of barasertib has been identified as 1200 mg when given as a 7-day infusion every 21 days to patients with AML. Overall, barasertib achieved a response in 25% of these patients with a manageable toxicity profile.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Stan Johnson from AstraZeneca for coordinating the execution of the study and contributing to the analysis, interpretation, and reporting of the study data; Drs Jane Robertson and Ozlem Ataman from AstraZeneca for providing medical support; Robert Smith from AstraZeneca for providing PK analyses; and Dr Juliet Fawcett from Mudskipper Bioscience for providing editorial assistance funded by AstraZeneca.
Authorship
Contribution: B.L. and H.K. conceived of and designed the study, acquired and interpreted the data, and drafted the manuscript; P. Sonneveld conceived of and designed the study and drafted the manuscript; P.M., G.O., P.R., J.-Y.C., N.I., G.M., E.B., P. Stockman, M.J.-L., and S.R. acquired the data; A.G., S.A., S.F., and E.J. analyzed and interpreted the data; and all authors critically reviewed, commented on, and approved the final manuscript.
Conflict-of-interest disclosure: B.L. is Chief Scientific Officer of and holds stock in Skyline Diagnostics and has equity in a Special Purpose Foundation governed by Erasmus University Medical Center. H.K. has received research grants from AstraZeneca. P. Stockman and A.G. are employees of and own stock in AstraZeneca. G.M. has an advisory role with Pfizer and has received honoraria from Novartis and BMS. The remaining authors declare no competing financial interests.
Correspondence: Bob Löwenberg, Erasmus University Medical Center, Main Building, Office L-413, Department of Hematology, PO Box 2040, Rotterdam 3000 CA, The Netherlands; e-mail: b.lowenberg@erasmusmc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal