In this issue of Blood, Neri and colleagues report a promising treatment of myeloma using a combination of bortezomib plus inhibition of the enzyme PARP1 (REF).1 This is welcome news, as new avenues for the treatment of the disease are needed.
Over the past decade we have seen new treatments available for patients; including proteasome inhibitors and the so-called immunomodulatory drugs. Combination strategies, often in conjunction with autologous stem cell transplantation, have maximized clinical efficacy of these drugs and some patients now enjoy durable remissions, including a minor subset that is cured from the disease.2 However, most patients relapse, some with high-risk genetic features relapse after only a short period of disease control.3 Improved strategies are needed.
Based on the seminal work done in triple negative breast cancer and ovarian cancers,4,5 Neri et al propose that inhibiting PARP may provide promise in the armamentarium against myeloma.1 The rationale for PARP inhibition is based on the observation that PARP1, a DNA damage repair enzyme, represents one of the last chances of cancer cells to undergo DNA repair and thus survive (see figure). Breast cancer patients with BRCA associated mutations (genotype) exhibit a large degree of genomic instability, the consequence of it genomic complexity on the form of DNA gains and losses (genotype-phenotype).6 This is particularly common in patients with triple negative breast cancer, where array-based comparative genomic hybridization analysis shows a high degree of genomic variance from normal (the so-called “BRCAness”).7 By inhibiting the repair activity of PARP, a safety net for DNA repair, investigators have been able to increase sensitivity of cancer cells to DNA-damaging agents. In contrast to solid tumors where PARP inhibitions leads to sensitization of cells to cytotoxic agents, Neri et al show that bortezomib renders myeloma sensitive to PARP inhibition.1
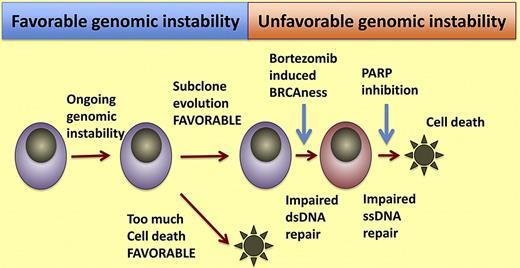
Synthetic lethal model for dual inhibition of single-stranded and double-stranded DNA repair. Cells that have some degree of genomic instability acquire genomic complexity that allows for favorable clonal selection and emergence of favorable genetic traits that confer proliferation, survival, or drug-resistant phenotypes. In a random stochastic process some of these cells suffer irreparable damage and cannot divide further. If this genomic instability is further increased such as the “BRCAness” induced in myeloma cells by bortezomib, it creates further challenges to myeloma cells. If subsequent inhibition of single-stranded DNA repair is inhibited by PARP inhibitors, Neri and colleagues are able to induce cell death.
Synthetic lethal model for dual inhibition of single-stranded and double-stranded DNA repair. Cells that have some degree of genomic instability acquire genomic complexity that allows for favorable clonal selection and emergence of favorable genetic traits that confer proliferation, survival, or drug-resistant phenotypes. In a random stochastic process some of these cells suffer irreparable damage and cannot divide further. If this genomic instability is further increased such as the “BRCAness” induced in myeloma cells by bortezomib, it creates further challenges to myeloma cells. If subsequent inhibition of single-stranded DNA repair is inhibited by PARP inhibitors, Neri and colleagues are able to induce cell death.
Neri and colleagues show PARP inhibition, after exposure to bortezomib, enhances the activity of both drugs. The key to their proposal is that bortezomib causes a BRCAness state, by virtue of the disruption of protein metabolism required for DNA repair. While ubiquitination is normally thought as focused on protein degradation, many nondegradation functions exist; including mediation of DNA repair mechanisms. This was achieved by diminishing the pool of ubiquitin and abrogation of H2AX polyubiquitylation, a necessary step in DNA repair. PARP inhibition with ABT-888 alone did not cause cell death, but when cells were pretreated with bortezomib PARP inhibition caused a greater degree of cytotoxicity than either drug alone. Normal cells can repair DNA breaks by the homologous recombination or the base excision repair mechanism. Cells that are deficient in homologous recombination as a consequence of BRCA mutations, or a BRCA state induced by bortezomib in the paper by Neri et al,1 are especially sensitive to DNA damage. It is then that the dual inhibition results in a synthetic lethal hit.
But why do cells die when both pathways are inhibited and no DNA stressor is added? That remains unanswered, although potential late effects of bortezomib are possible. Yet the potential clinical implications for these findings are remarkable. It is noteworthy that in other clinical settings PARP inhibition has worked best when used in combination with DNA-damaging agents. Myeloma provides a unique opportunity to further test this approach. Bortezomib combinations with alkylators have resulted in some of the best, if not the best, rates of response and clinical outcomes.8-10 A logical next step is using PARP inhibition plus bortezomib as well with bortezomib, alkylators, and PARP inhibitors. This approach could even further enhance cytotoxicity and lead myeloma cells to a “mitotic catastrophe.” If enough cells achieve this the permanent eradication of a clone could be possible, by pushing cells off a cliff of death.
Is there an ideal group of patients where this approach might be of greater help? Well, there is a body of emerging data that suggests that the level of genomic instability is greater in patients currently classified as having high-risk myeloma.6 This has been determined at the DNA level using high-density array-based comparative genomic hybridization and also using next-generation sequencing approaches.11 In fact, Neri et al show that elevated levels of PARP, presumptively trying to repair ongoing genomic instability, are associated with shortened survival, but likely because of its association with high-risk versions of myeloma and not in a causative fashion.1 A slight increase of ongoing genomic instability has evolutionary advantages for a clone and may be a key driver to more aggressive disease, by favoring subclonal diversity and emergence of more fit, proliferative, and ultimately drug-resistant clones. But if too much genomic instability occurs then these cells would risk a perilous outcome with the potential of genetic chaos and cell death. One could capitalize on this “meta-stability” and promote further instability, as with PARP inhibition of DNA repair, so that the clone could then become more susceptible to DNA-damaging agents. It is then that PARP inhibitors could become part of the armamentarium against high-risk myeloma or relapsed disease. While much progress has been made in the fight against myeloma, the utility of current agents is close to being maximized and new strategies are needed. Exploiting genomic instability against myeloma and as context lethality represents some of the most elegant science PARP excellence!
Conflict-of-interest disclosure: The author has received a patent for the prognostication of MM based on genetic categorization of the disease. He has received consulting fees from Medtronic, Otsuka, Celgene, Genzyme, BMS, Lilly, Millenium, and Amgen. He also has sponsored research from Cylene and Onyx. ■