Abstract
Myeloid antigen-presenting cells (APCs), regulatory cells, and the HLA-G molecule are involved in modulating immune responses and promoting tolerance. APCs are known to induce regulatory cells and to express HLA-G as well as 2 of its receptors; regulatory T cells can express and act through HLA-G; and HLA-G has been directly involved in the generation of regulatory cells. Thus, interplay(s) among HLA-G, APCs, and regulatory cells can be easily envisaged. However, despite a large body of evidence on the tolerogenic properties of HLA-G, APCs, and regulatory cells, little is known on how these tolerogenic players cooperate. In this review, we first focus on key aspects of the individual relationships between HLA-G, myeloid APCs, and regulatory cells. In its second part, we highlight recent work that gathers individual effects and demonstrates how intertwined the HLA-G/myeloid APCs/regulatory cell relationship is.
Introduction
Antigen-presenting cells (APCs) are specialized cells that process and present antigens (Ags) in the context of HLA class I and II molecules, and activate T cells. Professional APCs also provide a set of additional signals that modulate the activation of the responding cell. The most important professional APCs are dendritic cells (DCs), which play a major role as APCs in inducing adaptive immune responses and are critically involved in promoting and maintaining immunologic tolerance. Myeloid DCs, on which this review will focus, are also called conventional DCs. They initiate immune responses by capturing and processing Ags in peripheral tissues and transporting them to secondary lymphoid organs where they prime T and B cells.1 Conventional DCs in steady state or within a microenvironment enriched in anti–inflammatory mediators, or specialized subsets of DCs, named tolerogenic DCs, are involved in immune homeostasis and in promoting and maintaining peripheral tolerance through induction of regulatory T cells (Tregs).1
Tregs are key players in inducing and maintaining immune tolerance. During the past decade, immune modulation by Tregs has attracted increasing attention as one of the mechanisms underlying immune tolerance. Seminal works in the mid 1990s indicated that different subsets of Tregs exist that could control immune responses against self-, allo-, and non-harmful Ags.2,3 Although cells with regulatory function exist within all major T- and NK-cell subsets, most attention has been focused on Tregs within the CD4+ subset. Natural and adaptive Tregs were described.4 Natural Tregs arise from the thymus, and their suppressor function is strictly dependent on high expression of the transcription factor forkhead box P3 (FOXP3).4 Adaptive Tregs include: (1) IL-10–producing type 1 regulatory T (Tr1) cells3,5 and (2) TGF-β–secreting T helper 3 (Th3) cells,6 which both arise from naive T cells in the periphery after priming by DCs in a tolerogenic milieu; (3) induced FOXP3+ Tregs that can be generated from CD4+ T cells in the presence of TGF-β7 ; and (4) iTr35 cells that are generated in the periphery in an IL-35– and IL-10–dependent manner.8 In addition to CD4+ Tregs, CD8+CD28− T suppressor (Ts) cells are a specialized subset of CD8+ T cells with regulatory activities that act on APCs and render them tolerogenic by promoting up-regulation of immunoglobulin-like transcripts ILT3 and ILT4 (LILRB2). In turn, such tolerogenic DCs promote regulatory CD4+ Tregs in an allo-Ag–specific fashion.9
During the last decade, it has become evident that adaptive Tregs are primarily induced by tolerogenic DCs. Circulating and tissue resident tolerogenic DCs, which are characterized by the expression of higher levels of IL-10 and lower levels of IL-12 and costimulatory molecules, can prime T cells to become Tregs. Priming of T cells to become Tregs can also be achieved by specialized subsets of DCs, identified based on the expression of specific markers, such as CD103 in the gut, or langerin in the skin.1
Other regulatory cells
Alternatively activated macrophages or myeloid-derived suppressor cells are another important subset of tolerogenic APCs.10 Mesenchymal stem cells (MSCs), which are a rare population of pluripotent cells of the bone marrow, can also be viewed as regulatory cells because they are able to inhibit T-cell responses via cell-contact-mediated mechanisms and soluble factors that include soluble HLA-G and IL-10. In MSCs, HLA-G5 expression is linked to that of IL-10, and also required for MSC-mediated immunosuppression, including Treg induction.11
The HLA-G molecule is a nonclassic HLA class I tolerogenic molecule with tissue-restricted expression that functions through the inhibitory receptors ILT2 (LILRB1), ILT4, and KIR2DL4. HLA-G expression in physiologic conditions is restricted to fetal trophoblasts at the maternal-fetal interface and adult tissues, such as thymic epithelium, cornea, MSCs, and pancreatic islets. HLA-G has first been described as contributing to maternal-fetal tolerance, but because it can inhibit a broad array of immune cells, its relevance was later shown in pathologies, such as autoimmune diseases, transplantation, oncology, inflammatory diseases, and viral infections.12
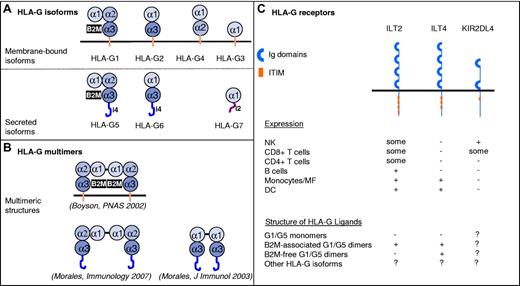
Structurally, HLA-G has 7 isoforms (4 membrane-bound, HLA-G1 to HLA-G4; and 3 soluble, HLA-G5 to HLA-G7) that are generated by alternate splicing of a unique primary transcript (Figure 1). Soluble and membrane-bound HLA-G isoforms have similar functions. B2M-associated HLA-G1 and HLA-G5 are structurally similar to classic HLA class I molecules. However, B2M-free HLA-G1/HLA-G5 molecules exist and may be of particular relevance in vivo: cytotrophoblast cells express B2M-free HLA-G5 molecules in vivo,13 and B2M-free HLA-G forms are particularly abundant in supernatant from tumor cell lines naturally expressing HLA-G.14 HLA-G possesses 2 unique cysteine residues: in position 42 (α-1 domain) and in position 147 (α-2 domain). Through these residues, HLA-G may dimerize by intermolecular disulfide bonds. Membrane-bound or soluble HLA-G dimers were detected in vitro and in vivo.15,16 Dimerization of HLA-G is one of its key features because dimers, but not monomers, carry most of HLA-G function.17
Structures and receptors of the HLA-G molecule. (A) The alternate splicing of a unique primary transcript yields 7 protein isoforms: truncated isoforms are generated by excision of 1 or 2 exons encoding globular (α) domains, whereas translation of intron 4 (i4) or intron 2 (i2) yields soluble isoforms that lack the transmembrane domain. (B) HLA-G molecules can form homodimers through the generation of Cys42-Cys42 disulfide bonds. Reported multimeric structures are presented and referenced. (C) Inhibitory receptors known to bind HLA-G. Basic structural organization and expression patterns are shown. The HLA-G structural configuration that these receptors are known to bind are indicated as follows: − indicates reported absence of binding or minor binding; +, reported binding; and ?, not reported.
Structures and receptors of the HLA-G molecule. (A) The alternate splicing of a unique primary transcript yields 7 protein isoforms: truncated isoforms are generated by excision of 1 or 2 exons encoding globular (α) domains, whereas translation of intron 4 (i4) or intron 2 (i2) yields soluble isoforms that lack the transmembrane domain. (B) HLA-G molecules can form homodimers through the generation of Cys42-Cys42 disulfide bonds. Reported multimeric structures are presented and referenced. (C) Inhibitory receptors known to bind HLA-G. Basic structural organization and expression patterns are shown. The HLA-G structural configuration that these receptors are known to bind are indicated as follows: − indicates reported absence of binding or minor binding; +, reported binding; and ?, not reported.
Unlike classic HLA class I molecules, the functions of HLA-G are exclusively oriented toward immune inhibition and tolerance. HLA-G acts through the inhibitory receptors ILT2, ILT4, and KIR2DL4. ILT2 and ILT4 recognize other HLA class I molecules, but HLA-G is their ligand of highest affinity and they recognize HLA-G dimers even more strongly. ILT2 recognizes only B2M-associated HLA-G1/HLA-G5 isoforms, whereas ILT4 also recognizes their B2M-free counterparts.18 ILT2 and ILT4 are differentially expressed by NK cells (ILT2, KIR2DL4), CD4+ (ILT2) and CD8+ (ILT2) T cells, B cells (ILT2), monocytes/macrophages (ILT2, ILT4), and myeloid DCs (ILT2, ILT4). HLA-G inhibits the cytolytic functions of uterine and peripheral blood NK cells, the antigen-specific cytolytic functions of cytotoxic T lymphocytes, the allo-response of CD4+ T cells, the proliferation of T and peripheral blood NK cells, and the maturation and functions of DCs. In addition to these direct immunoinhibitory functions, HLA-G has long-term tolerogenic functions by inducing Tregs.12,19
Physiologically, HLA-G has first been described to be a key player in promoting maternal-fetal tolerance. Later, because it can inhibit a broad array of immune cells, its relevance was shown for pathologies, such as autoimmune diseases, transplantation, oncology, inflammatory diseases, and viral infections.12 The expression of HLA-G may be beneficial in the contexts of pregnancy and transplantation, in which it protects fetuses and transplants from immune destruction, possibly through the induction of Tregs and tolerogenic DCs. However, HLA-G expression may also be detrimental when it promotes tolerance to tumors or virus-infected cells.
Individual interactions between HLA-G, myeloid APCs, and regulatory cells
Because HLA-G, myeloid APCs, and regulatory cells are all involved in immune inhibition and tolerance, their relationships were investigated. Most of the data concern individual interactions between 2 of these 3 players and focused on: (1) the functions of HLA-G on myeloid APCs; (2) the expression of HLA-G by myeloid APCs; and (3) the capability of HLA-G to characterize and/or induce regulatory cells.
The functions of HLA-G on myeloid APCs
The function of HLA-G on myeloid APCs has first been hypothesized when the ILT2 and ILT4 receptors were characterized and their cellular localization known. Indeed, these receptors bind HLA-G on one hand and are inhibitory on the other. Given the myeloid-specific expression of ILT4, it was postulated that this receptor could modulate one or several of the antigen-presenting functions of myelomonocytic cells, such as Ag uptake and presentation, migratory capacity, cytokine production, and costimulatory expression.20 This hypothesis was strengthened by data showing that tetramers of HLA-G–bound ILT4 transfectants more efficiently than ILT2 transfectants. Furthermore, these tetramers stained only PBMC monocytes through binding to ILT4, and not lymphocytes,21 hinting that myeloid APCs may be the cell type most involved in the in vivo functions of HLA-G, and the only one capable of reacting to the full spectrum of HLA-G structures.
In the presence of HLA-G, myeloid APCs failed to stimulate alloproliferative T-cell responses in vitro.19 Similarly, treatment of monocyte-derived DCs by soluble HLA-G altered the DC/NK-cell cross-talk, leading to reduced NK-mediated cytotoxicity.22 Moreover, HLA-G inhibited the up-regulation of HLA class II and costimulatory molecules, such as CD80 and CD86, on myeloid DCs in response to lipopolysaccharide or alloactivation signals in vitro.22-24 Thus, results in vitro suggested that HLA-G inhibited the functions and differentiation of myeloid APCs, leading to improper T lymphocyte activation and to impaired NK cytotoxic activity. Results obtained in a murine model supported this notion by showing that: (1) HLA-G inhibited the maturation of immature DCs into functionally competent mature DCs25 ; and (2) in ILT4-transgenic mice, the HLA-G–ILT4 interaction impaired DC maturation in vivo, leading to delayed skin allograft rejection.26
However, it soon became evident that HLA-G does not block myeloid APCs but induces them to take an alternative differentiation path. The first evidence came from reports showing that HLA-G treatment altered the expression of cytokines, chemokines, and chemokine receptors by myeloid APCs.16,22,27,28 HLA-G–induced IL-6 and IL-10 up-regulation, for instance, is a finding that is not consistent with a mere functional blockade of APCs. Thus, this constituted the first hint that HLA-G positively acts on myeloid DCs and promotes an alternative differentiation path.
The direct proof that HLA-G induces tolerogenic APCs came after it was shown that ILT4 transduction into myeloid cell lines rendered them tolerogenic9 and that ILT3 and ILT4 overexpression is a feature of tolerogenic myeloid DCs.29 It was first shown that monocyte-derived DCs that highly express ILT2 and ILT4 receptors maintain a stable tolerogenic-like phenotype (CD80low, CD86low, HLA-DRlow) with the potential to induce T-cell anergy, when treated with HLA-G and stimulated with allogeneic T cells.30 Furthermore, in mixed lymphocyte reactions, the proliferation of allogeneic T cells was inhibited by the binding of HLA-G to ILT2/ILT4 on responding APCs, not on effector T cells.16 Moreover, in an ILT2-transgenic murine model, it was clearly demonstrated that the HLA-G/ILT2 interaction promoted the differentiation of myeloid-derived suppressor cells capable of significantly prolong skin allograft.31 Thus, HLA-G, by interacting with ILT receptors present on myeloid APCs, induces their differentiation into regulatory cells.
Myeloid APCs that express HLA-G
Myeloid APCs may not only be viewed as the cells most sensitive to HLA-G, but also as cells that commonly express it. They may express all HLA-G isoforms,32 but cell-surface HLA-G1 and secreted HLA-G5 have been described the most. The basal expression of HLA-G by myeloid cells is greatly enhanced by microenvironmental factors, such as interferons33 and IL-10,34,35 and by maturation stimuli.32 Immunotolerogenic stimuli, such as CTLA4-Ig, may further enhance HLA-G up-regulation.36 Interestingly, the tryptophan catabolyzing enzyme indoleamine 2 3-dioxygenase (IDO) was shown to differentially modulate HLA-G expression in monocytes and myeloid DCs: IDO blocked HLA-G cell-surface expression on monocytes,37 but it induced HLA-G expression and shedding in myeloid DCs.38 IDO is known to be involved in the generation of tolerogenic microenvironments by tryptophan depletion and in the generation of Tregs.39,40 Thus, HLA-G and IDO, when produced by myeloid DCs, may cooperate and promote immunotolerance.
Myeloid cells with up-regulated HLA-G expression were detected in pathologic contexts, such as transplantation, cancer, viral infections, and inflammatory diseases.12 In liver-transplanted patients, the presence of HLA-G–expressing myeloid DCs correlated with tolerance and graft acceptance.41 In cancer, myeloid APCs expressing HLA-G were detected within breast, lung, and ovarian carcinoma lesions as well as in melanoma, and often correlated with a poor clinical outcome.42 In viral infections, the expression of HLA-G by myeloid APCs was reported for HIV and human cytomegalovirus and considered a viral immune escape mechanism.43
HLA-G+ myeloid APCs may secrete or shed HLA-G molecules, and so contribute to the generation of a tolerogenic microenvironment. Such a microenvironment may alter the functions not only of lymphocytes, but also of the HLA-G–expressing myeloid APCs themselves, in a tolerogenic feedback loop. Thus, myeloid APCs that express HLA-G may be viewed as suppressor cells capable of inhibiting other immune effectors and also of generating regulatory cells, such as Tregs.19 Myeloid APCs that express membrane-bound HLA-G may also constitute a source of HLA-G–containing membranes that can be acquired by activated lymphocytes through the mechanism of trogocytosis, turning them into temporary regulatory cells.44 Thus, HLA-G+ myeloid APCs play a pivotal role in promoting a tolerogenic milieu that may be beneficial in transplantation and autoimmune diseases but detrimental in other conditions, such as cancer and viral infections.
HLA-G and regulatory cells
HLA-G is immunoinhibitory by itself. This implies that cells expressing HLA-G may use it to inhibit functions of immune effector cells. This was first demonstrated when nonantigenic K562 cells expressing HLA-G1 were added as third party cells to a mixed lymphocyte reaction and inhibited CD4+ T-cell allo-proliferation.45 The immunoinhibitory function mediated by HLA-G has been confirmed for APCs,19 tumor cells,46 and T and NK cells, whether they express HLA-G endogenously47 or acquire it by membrane transfers from other cells (trogocytosis).44
Atop direct inhibition of effector cells, HLA-G is known to induce bona fide Tregs: in the absence of stimulation, PBMCs that had been pretreated with soluble HLA-G5 acquired regulatory properties, and they inhibited allo-proliferative responses of other T cells.48 These results are in line with those obtained in patients who received a combined liver-kidney transplant, in which high plasma levels of HLA-G5 correlated with an increased percentage of suppressor T cells.48,49 They are also in agreement with those showing that high HLA-G5 plasma levels in the peripheral blood of stem cell–transplanted patients are associated with the expansion in peripheral blood of CD4+CD25+CD152+ T cells with suppressive activity.50 Soluble HLA-G up-regulated the expression of ILT2, ILT3, and ILT4 by lymphocytes and myeloid cell lines.51 It is not known whether these HLA-G–treated cells were regulatory cells, but this is a possibility because ILT3 and ILT4 up-regulation is a hallmark of myeloid regulatory cells.29 Thus, HLA-G promotes not only Tregs but also regulatory APCs.
Membrane-bound HLA-G1 was also shown to characterize and/or induce Tregs. Indeed, naturally occurring CD4+ Tregs expressing membrane-bound HLA-G1 (CD4+HLA-G+ T cells) have been described. These cells are present in both peripheral blood and the thymus of healthy persons47 and are enriched at sites of inflammation.52 CD4+HLA-G+ T cells not only express membrane-bound HLA-G1 but also secrete soluble HLA-G5,47 which, in association with IL-10, suppresses T-cell responses in vitro.52 Recently, induced CD4+HLA-G+ T cells that participate in the induction of Tregs by DCs in an IL-10–dependent fashion were also reported.34
Finally, HLA-G+ APCs were shown to be tolerogenic cells capable of priming naive T cells to become Tregs. Indeed, APC lines overexpressing membrane-bound HLA-G1 induced the differentiation of CD4+ and CD8+ T cells able to inhibit allogeneic responses.19 Resulting HLA-G-induced Tregs included CD4low and CD8low T cells that suppress via soluble factors.49 Recently, a new subset of DCs that arises in the presence of IL-10 and endogenously expresses cell-surface HLA-G was characterized (see next paragraph).34
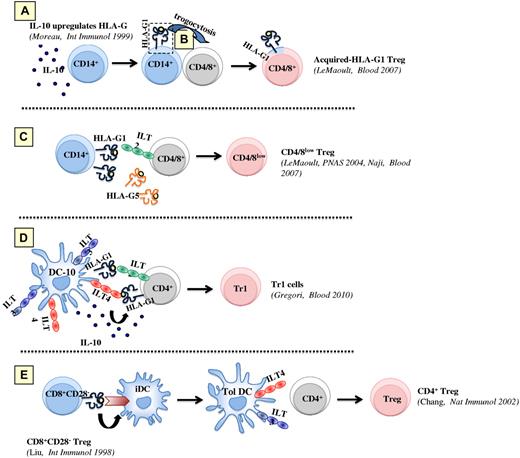
Thus, individual relationships between HLA-G, myeloid APCs, and regulatory cells have been evidenced, which are summarized in Figure 2. Taken together, these data suggest that HLA-G may be involved at all steps of cell-mediated tolerance: as cellular product of APCs and regulatory cells, as inducer of their differentiation, and as effector molecule of their function. Few reports went beyond individual relationships: these showed that HLA-G–treated myeloid APCs are able to induce Tregs in mice and human beings,23,27 that HLA-G–expressing APCs prime T cells to become Tregs in vitro,19 and that the level of HLA-G on DCs correlates with Treg incidence in liver-transplanted patients in vivo.41 Recently, the question of APCs/HLA-G/regulatory cell interactions was addressed in a more integrated fashion.34
HLA-G/APCs/regulatory cell known interactions, including DC-10. Tolerogenic mechanisms that are dependent on HLA-G–expressing monocytes or DC-10 and that may cooperate to create a tolerogenic milieu enriched in IL-10 and soluble HLA-Gs, and induce Tregs. (A) IL-10 present in the microenvironment promotes the up-regulation of membrane-bound HLA-G1 on APCs, including monocytes. (B) After a short interaction with HLA-G1–expressing APCs, CD4+ or CD8+ T cells (and NK cells, not shown) acquire HLA-G1–containing membrane fragments (trogocytosis) and become temporary Tregs. (C) Both soluble and membrane-bound HLA-G expression on APCs induces Tregs, some of which are characterized by a CD4low and CD8low phenotype. (D) In the presence of IL-10 secreted by DC-10, the interactions between ILT4 and HLA-G1 on DC-10, and HLA-G1 and ILT2 on T cells, respectively, promote the induction of Tr1 cells. (E) CD8+CD28− T cells engage HLA class I on APCs and induce tolerogenic DCs, which overexpress ILT3 and ILT4.
HLA-G/APCs/regulatory cell known interactions, including DC-10. Tolerogenic mechanisms that are dependent on HLA-G–expressing monocytes or DC-10 and that may cooperate to create a tolerogenic milieu enriched in IL-10 and soluble HLA-Gs, and induce Tregs. (A) IL-10 present in the microenvironment promotes the up-regulation of membrane-bound HLA-G1 on APCs, including monocytes. (B) After a short interaction with HLA-G1–expressing APCs, CD4+ or CD8+ T cells (and NK cells, not shown) acquire HLA-G1–containing membrane fragments (trogocytosis) and become temporary Tregs. (C) Both soluble and membrane-bound HLA-G expression on APCs induces Tregs, some of which are characterized by a CD4low and CD8low phenotype. (D) In the presence of IL-10 secreted by DC-10, the interactions between ILT4 and HLA-G1 on DC-10, and HLA-G1 and ILT2 on T cells, respectively, promote the induction of Tr1 cells. (E) CD8+CD28− T cells engage HLA class I on APCs and induce tolerogenic DCs, which overexpress ILT3 and ILT4.
DC-10 and the complex interplay between HLA-G, APCs, and regulatory cells
A new subset of human tolerogenic DCs, named DC-10 for its outstanding ability to produce IL-10, has been recently identified and characterized.34 DC-10 are present in vivo in peripheral blood and secondary lymphoid organs, are inducible in vitro from monocytes in the presence of IL-10, and are characterized by the expression of high levels of membrane-bound HLA-G1 and other tolerogenic signaling molecules, such as ILT2, ILT3, and ILT4. DC-10 are potent inducers of adaptive allo-specific Tr1 cells.34 Furthermore, allergen-specific Tr1 cells can be generated in vitro by stimulating human T cells with autologous tolerogenic DC-10 pulsed with allergen.53 Interestingly, the expression of membrane-bound HLA-G1 and that of its receptors is up-regulated by IL-10 on both DC-10 and T cells, and the expression of high levels of membrane-bound HLA-G1, ILT4, and IL-10 by DC-10 is critical to the generation of Tr1 cells by DC-10.54
Based on these findings, it has been postulated that HLA-G and IL-10 generate a tolerogenic loop: first, IL-10 up-regulates the expression of HLA-G and its receptors on both DCs and T cells. This increases the secretion of IL-10 and possibly of soluble HLA-G by DCs, which in turn promotes the differentiation of Tr1 cells. By secreting IL-10 themselves, Tr1 cells favor the de novo expression of HLA-G and its receptors by neighboring DCs.1,34 The role of membrane-bound HLA-G1 in promoting Tr1 cells via DC-10 raised the question of whether soluble HLA-G molecules, such as shed sHLA-G1, can also promote Tr1 cell differentiation. Interestingly, we showed in vitro that stimulation of CD4+ T cells in the presence of shed sHLA-G1 alone or in combination with IL-10 induced the differentiation of a population of CD4+ T cells that suppress proliferation of autologous CD4+ T cells (C. F. Magnani, M. G. Roncarolo, and S.G., unpublished data, March 2009) but are phenotypically and functionally different from Tr1 cells.5 In particular, suppressor CD4+ T cells generated by stimulation in the presence of soluble HLA-G did not secrete IL-10. The inability of suppressor T cells generated with shed sHLA-G1 to secrete IL-10 can be explained by the lower HLA-G1–mediated signaling in T cells and by the absence of ILT4-mediated signaling in myeloid APCs.55
The discovery of HLA-G–expressing DC-10 and their role in promoting tolerance via adaptive Tregs34 offers the possibility to reconcile the immunomodulatory circuits mediated by HLA-G and places HLA-G as central molecule in promoting a tolerogenic microenvironment. For instance, at the fetal-maternal interface, (1) trophoblasts are the main cell subset expressing HLA-G, and (2) by interacting with maternal APCs in a microenvironment enriched in IL-10 and progesterone, they drive the conversion of APCs into tolerogenic cells. (3) Given that decidual macrophages express CD14, CD163+, and both ILT2 and ILT4 receptors,56 a phenotype that matches that of DC-10 cells, it is possible that they are DC-10 or DC-10–like cells.
Similarly, during CMV or HIV infections, increased serum levels of soluble HLA-G and up-regulation of membrane-bound HLA-G1 expression by monocytes were repeatedly reported, and it was otherwise shown that the interaction between soluble HLA-G and ILT4 on DCs from HIV-infected patients leads to low functional activity of DCs.24 Moreover, the high plasma IL-10 levels in HIV-infected patients were shown to promote the induction of both tolerogenic DCs and IL-10–producing Tr cells in vivo.57 Thus, it is possible that HIV, by promoting IL-10, not only increases the release of intracellular reservoirs of HLA-G by myeloid cells (monocytes and DCs)24 but also induces the up-regulation of membrane-bound HLA-G1, ILT2, ILT3, and ILT4 on DCs, and the differentiation of DC-10 or DC-10–like cells, which in turn induce IL-10–producing Tr cells.57
Thus, in both these examples, it is very possible that myeloid APCs, HLA-G, IL-10, and regulatory cells, which have been independently investigated, are all key components of a general tolerogenic mechanism centered around the HLA-G/DC interaction.
In conclusion, significant progress has been made in understanding the interaction between HLA-G, myeloid APCs, and regulatory cells. These mechanisms provide compelling evidence that HLA-G is a molecule that may be key to tolerance induction, as inducer of regulatory cell differentiation, and/or as tolerogenic molecule. The recent discovery of DC-10 cells demonstrates that HLA-G may perform all these individual functions contemporaneously. More work is now needed to validate this hypothesis. In particular, the parameters of the interactions between HLA-G and its receptors at the surface of myeloid APCs are poorly defined. When HLA-G/ILT4 interaction is crucial, such as in the context of DC-10, it is necessary to precisely characterize the mechanisms by which ILT4 signaling yields to DC-10 generation and function. Similarly, because ILT receptors are up-regulated by tolerogenic myeloid APCs and possibly Tregs, it is very important to determine how HLA-G affects regulatory cells and tolerogenic DCs. HLA-G may promote tolerogenic DCs and Treg cell survival, proliferation, or function, but it is equally reasonable that ILT, being inhibitory receptors, HLA-G may inhibit the overall function of tolerogenic cells. It is also possible that HLA-G does both: being a structurally complex molecule, different HLA-G conformations may have different functional outcomes. Thus, it is necessary to investigate which HLA-G structures are actually present in vivo and which HLA-G domains mediate HLA-G function.
Overall, the data already available indicate that HLA-G acts at several levels and promotes the generation of multiple regulatory cells (HLA-G–acquired Tregs,44 CD4/8low suppressor cells,19,49 and Tr1 cells,34 ), which cooperate, in association with other additional Tregs, in promoting tolerance networks. It is now of critical importance that the tolerogenic function of HLA-G through myeloid APCs and regulatory cell generation be taken into account in therapeutic strategies, especially in the context of oncology, which involves myeloid APCs and vaccinations, such as the treatment with anti–CTLA-4. Indeed, if HLA-G is present, it may block the generation and/or function of effector T cells and render a cytotoxic T cell–based treatment ineffective. Worse, the combination of Ag immunization and HLA-G may start the induction of tolerogenic DCs which, as reported, induce Ag-specific Tregs, thus inducing tumor tolerance instead of restoring tumor Ag-specific immunity. This does not mean that these approaches are ineffective but that a molecule such as HLA-G, which is central to some tolerogenic networks, should be considered in the design of therapeutic strategies. Concomitant treatments that block or inactivate HLA-G should be considered.
Acknowledgments
This work was supported by CEA, the Telethon Foundation, the Associazione Italiana per la Ricerca sul Cancro, and the Italian Ministry of Health.
Authorship
Contribution: E.D.C., S.G., and J.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edgardo D. Carosella, CEA-Service de Recherches en Hemato-Immunologie, Instituto Universitare d'Hemalologie, Hopital Saint Louis, 1 Avenue Claude Vellejause, 75475 Paris Cedex, France; e-mail: edgardo.carosella@cea.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal