The 2011 Nobel Prize in Physiology or Medicine was awarded to Ralph M. Steinman, Jules Hoffman, and Bruce Beutler for their discovery of dendritic cells (DCs) and Toll-like receptors (TLRs). Their research paved the way for harnessing DCs to create vaccines.
An effective vaccine contains immunodominant antigens and adjuvants that are processed by in vivo DCs to elicit humoral and cellular (effector and memory) immunity. Uncoupling DCs and their adjuvants via route or scheduling of administration may reduce or eliminate the desired immune response. In this issue of Blood, Tacken et al show for the first time that in vivo DC vaccination benefits from co-targeting antigen with adjuvants via nanometer sized particulate carriers (see figure).1
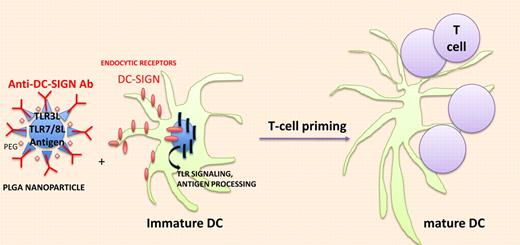
In vivo targeting of DCs with nanodevice containing immunodominant antigens and TLR agonists. Biodegradable poly (lactic-coglycolic) acid (PLGA) nanoparticles (NPs) effectively deliver antigen and TLRLs to DCs and trigger endocytosis through C-type Lectin receptors and accelerate antigen processing and presentation through TLR signaling.
In vivo targeting of DCs with nanodevice containing immunodominant antigens and TLR agonists. Biodegradable poly (lactic-coglycolic) acid (PLGA) nanoparticles (NPs) effectively deliver antigen and TLRLs to DCs and trigger endocytosis through C-type Lectin receptors and accelerate antigen processing and presentation through TLR signaling.
Steinman described DCs as the mandatory component linking antigen and immunogenicity. Early clinical trials demonstrated the safety and immunogenicity of autologous DCs loaded with exogenous antigens, yet revealed a defect in the migratory pathways of these cells and inconsistent clinical benefit against cancer. The recent FDA approval of Sipuleucel-T (Dendreon) admixing antigen presenting cells exposed to a fusion protein (composed of a prostate specific antigen and GM-CSF) to autologous lymphocytes in prostate cancer rekindled the interest of harnessing DCs for vaccine development. However, a more direct strategy using DCs residing in lymphoid organs has been investigated over the past 10 years.
The great potential for targeting antigen presenting cells (APCs) with antibody-opsonized antigens to induce humoral immune responses was brought up decades ago using anti-FcγR or MHC antibodies. Lately, the identification of DC subsets and their specific surface receptors as well as recent advances in understanding antigen capture, processing, and presentation have stimulated research on DC targeting in vivo. The quality of the immune response elicited by in vivo DC vaccination will depend on the expression pattern and biologic properties of the targeted receptor, the activation status of the DCs, and the formulation of the antigen. Numerous studies performed in mouse models demonstrated the efficacy of targeting plasmacytoid DCs, CD8α−DCIR2+ DCs or CD8α+CD103+ DCs, or epidermal Langherans cells with antibodies directed against C-type lectin endocytic receptors (Siglec-H, DCIR2, DEC205, or Clec9A, Langerin, respectively) to induce humoral and/or cellular CD4+ and/or CD8+ T-cell responses. In the absence of adjuvants, targeting DEC205+ DCs in vivo can induce tolerance.2 Depending on the DC subtype more specifically targeted by the antibody, one might favor the use of anti-CD40 agonistic antibodies and/or TLR3 or TLR7/8 agonists to promote the concomitant maturation of engulfing DCs.3
The first phase 1 trials assessing in vivo DC-targeting vaccines delivered antigen (human chorionic gonadotropin [hCG-b]) to APCs by antibody-mediated targeting of a mannose receptor. Delivery of this product with GM-CSF and TLR3/TLR7/8 agonists induced consistent humoral and cellular immune responses to hCG-b. However, this study indicated stronger immune responses and longer durations of disease stability when the antibody was co-administered locally with TLR agonists.4 Uncoupling DCs and their adjuvants may not represent an ideal strategy. For safety reasons, adjuvants should not be given systemically. Conversely, antibody-mediated targeting of in vivo DCs should be disseminated enough to reach lymph node–resident DCs that will mount T-cell responses capable of recirculating. Indeed, activated effector/memory T cells migrate preferentially to tissues that are draining the secondary lymphoid organs where antigen was first encountered.
Blander and Medzhitov reported that the efficacy of presenting antigens from phagocytosed cargo is dependent on the presence of TLR ligands within the cargo (reviewed in Tacken and Figdor3). This is due to MHC class II–restricted antigen presentation being governed by TLR signaling occurring selectively in phagosomes. Indeed, conjugating antigens with TLR agonists enhanced the magnitude and quality of Th1 and CD8+ T-cell responses in nonhuman primates.5 The next step was to successfully target such antigen/TLR conjugates to DCs. Therefore, Figdor et al undertook the packaging of adjuvants and antigens within particulate vaccine vehicles carrying DC-specific antibodies on their surface.8 Nanometer-sized (as opposed to micrometer-sized) particulate vaccine carriers recirculate in the lymphatics, show high tissue penetration, and trigger humoral and cellular immune responses.6-8
Tacken et al pursued their work encapsulating TLR3 and TLR7/8 ligands (TLRLs; PolyI:C+R848) within targeted biodegradable poly (lactic-coglycolic) acid nanoparticles (NPs) that effectively deliver antigen to human DCs in vitro (see figure). NPs were surface-coated with lipid PEG to limit nonspecific interactions with cells and armed with anti–DC-SIGN–specific humanized IgG1/IgG4 antibodies. Within 1 hour, most monocyte-derived DCs (MDDCs) had taken up DC-SIGN–targeted NPs while isotype control Ab NP was detectable at 20 hours. As for induction of maturation, DC-SIGN–targeted NPs were 100-fold more potent to induce CD80 and CD83 expression as well as cytokine release (TNFa, IL-6, IL-12p70) from immature MDDCs than soluble TLR agonists. These findings translated into a 100-fold enhanced capacity of the DC-SIGN–targeted MDDCs loaded with TLR ligands encapsulating NPs and pulsed with tumor peptides to stimulate tumor-specific TCR transgenic CD8+ T cells to produce Th1 cytokines (compared with immature MDDCs pulsed with uncoupled peptides and TLR agonists). To further evaluate the immunogenicity of antibody-coated NPs loaded with antigens and TLR agonists in mouse models, Tacken et al had to select another C-type lectin, that is, DEC205, which is expressed on the cell surface of spleen and LN DCs, in contrast to mouse DC-SIGN (which remains internalized in mice). F(ab′)2 fragments (to avoid internalization via FcgR) of an anti-DEC205 Ab were coated onto the NPs encapsulating the OVA candidate antigen and TLR3 and TLR7/8 agonists. Such DEC205-targeted NPs could easily bind to mouse DCs and stimulate OT-1–specific T cells in vitro. In vivo cytotoxicity assays revealed that only NP-DEC205-OVA-TLRL–treated mice could mount an antigen-specific CTL response. Littermates treated with NP-DEC205-OVA + soluble TLRLs or NP-isotype control Ab-OVA-TLRLs failed to mount a response. Increasing the dosing of soluble TLRLs by 100-fold rendered the control mice treated with NP-DEC205-OVA competent to elicit a peptide-specific CTL response but this was accompanied by severe side effects (shock-like symptoms associated with high type 1 IFN serum levels). These data are in line with a previous report targeting the delivery of a tumor antigen and LPS within liposomes coated with single-chain antibody directed against mouse CD11c or DEC205, a technology resulting in a survival benefit of cancer-bearing mice.9
In vivo targeting of DCs with nanodevice containing immunodominant antigens and TLR agonists appears ideal in that a single GMP product with large-scale manufacturing could be obtained at low cost and be accessible to large numbers of patients. The well-known coordinated action of the different DC subsets may require simultaneous injections of various antibody-coated NPs containing different TLRLs and/or antigens. Work needs to be done to restrict the specificity of the targeting10 and controlling the maturation status of tissue-residing DCs.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal