In this issue of Blood, Amir et al report how donor T cells recognize major histocompatibility complex (MHC) alloantigens of the recipient in a patient with graft-versus-host disease (GVHD).1
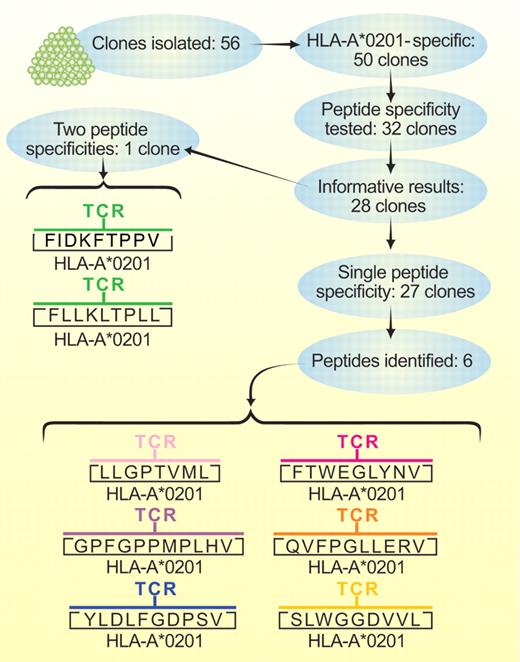
Flow of work by Amir et al.1 Peptide specificities presented in conjunction with HLA-A*0201 were identified for the clone with 2 specificities and for 6 of the clones with a single specificity. Experiments with silencing hairpin RNA sequences that suppressed specific gene expression confirmed that no other peptides were recognized by 4 of the 6 clones with single peptide specificities or by the clone with 2 peptide specificities. Two of the clones that recognized a single peptide were not tested in this type of experiment. Professional illustration by Paulette Dennis.
Flow of work by Amir et al.1 Peptide specificities presented in conjunction with HLA-A*0201 were identified for the clone with 2 specificities and for 6 of the clones with a single specificity. Experiments with silencing hairpin RNA sequences that suppressed specific gene expression confirmed that no other peptides were recognized by 4 of the 6 clones with single peptide specificities or by the clone with 2 peptide specificities. Two of the clones that recognized a single peptide were not tested in this type of experiment. Professional illustration by Paulette Dennis.
During development in the thymus, T cells are selected to survive when the T-cell receptor (TCR) has weak affinity for self-MHC molecules, so that in the periphery, immune responses are triggered when foreign antigen-derived peptides are inserted into the groove between the α helices of self-MHC molecules and strengthen the interaction with the TCR above a critical threshold. In this way, T cells are “biased” toward recognition of peptide antigens presented by self-MHC molecules. This understanding raised questions of whether T cells respond to MHC alloantigens primarily by recognizing polymorphic differences between allo-MHC molecules per se, with relatively little contribution from the peptide, or by recognizing foreign peptides in a highly restricted way that somehow reflects the bias toward self-MHC molecules.2
A previous study showed that a human T-cell clone specific for an Epstein-Barr virus–derived peptide in conjunction with self–HLA-B8 molecules could also recognize as many as 4 distinct human-derived peptides in conjunction with HLA-B35, suggesting that MHC-polymorphism has a dominant role in allo-MHC recognition.3 In another study, however, 3 human T-cell clones specific for HLA-A*0201 each recognized unique, single, distinct peptide peaks in experiments where T-cell recognition was reconstituted with fractions isolated by reverse-phase, high-performance liquid chromatography (HPLC), suggesting that the peptide plays a critical role in allo-MHC recognition.4 A more recent study of murine T-cell clones specific for an MHC–class II alloantigen showed mixed results: 3 clones responded to 2 or 3 different peptides inserted into the groove between the α-helices, whereas 6 clones appeared to be specific for a single peptide.5
Amir et al isolated 56 T-cells clones from a patient with GVHD after hematopoietic cell transplantation from a related donor.1 The related donor and recipient were HLA genotypically identical except at HLA-A. Because of a recombination between HLA-A and -B, the recipient was HLA-A*0201-positive, whereas the donor was HLA-A*0201-negative, thereby creating a situation in which the donor T-cell response was driven by recognition of HLA-A*0201 in the recipient.
Fifty of the clones recognized HLA-A2 (see figure). Thirty-two of these clones were tested to determine whether T-cell recognition could be reconstituted by loading HPLC fractions of peptides isolated from HLA-A*0201 molecules into HLA-A*0201-positive human T2 cells that are not able to insert peptides into MHC class 1 molecules through a transporter associated with antigen processing or by loading HPLC fractions into HLA-A*0201-positive insect cells that lack peptides derived from human molecules other than HLA-A*0201. In 27 of the 28 informative assays, the clones each responded to a single unique peptide, and only 1 clone responded to 2 distinct peptides. Six of the single peptides recognized by a clone and both peptides recognized by the clone with dual specificity were identified by mass spectrometry. Experiments with silencing short hairpin RNA sequences that suppressed expression of specific genes encoding 6 of the 8 identified peptides confirmed that these T-cell clones recognized no other peptides in conjunction with HLA-A*0201.
These results show that unlike minor histocompatibility antigens composed of a single specific peptide presented by a specific MHC molecule, major histocompatibility antigens represent large families of single specific peptides that can all be presented to T cells by a specific MHC molecule. The response to a single MHC alloantigen has been estimated to be approximately 3 orders of magnitude stronger than the response to any single minor histocompatibility antigen. These results also show why the response to an MHC alloantigen overwhelms any concurrent response against minor histocompatibility antigens that would otherwise have been generated in the absence of an MHC mismatch.
Amir et al also showed that the T-cell clones did not recognize a panel of virus-derived peptides presented by HLA-A*0201.1 A more interesting question is whether any of the clones recognize virus-derived peptides presented by other MHC molecules in the donor, because previous studies by the same group indicated that at least 45% of T cells specific for virus-specific peptides presented by self-MHC molecules also recognize MHC alloantigens.6 Mechanisms that permit such diverse cross-reactivity have recently been elucidated by studies comparing the crystal structures of a TCR bound to a peptide-specific member of an MHC alloantigen family or bound to a virus-derived peptide in conjunction with a different MHC molecule. These mechanisms include molecular mimicry, altered docking of the TCR with the peptide-MHC ligand, conformational changes in the MHC molecule induced by peptide binding, and conformational changes in either the TCR or the peptide-MHC ligand induced during the ligand-receptor interaction.7
The polyspecific nature of TCR recognition has raised concerns that the use of viral or tumor-associated peptide-specific T cells for adoptive immunotherapy could cause GVHD in MHC-mismatched recipients.8,9 Despite these concerns, a recent report by Melenhorst et al showed no exacerbation of GVHD in patients treated with virus-specific T cells that cross-reacted with a recipient MHC alloantigen in vitro.10 Several explanations could account for these unexpected results. It is possible, for example, that the peptide components of the MHC alloantigens were not expressed in GVHD target tissues of the recipients or that the virus-specific T cells were derived primarily from an effector memory population that has an intrinsically limited ability to cause GVHD.11
Production of therapeutic T cells specific for an individual patient takes at least several weeks. More timely treatment would be feasible if ready-made “off-the-shelf” cellular reagents that recognize viral or tumor-associated peptides of interest in the context of specific MHC types that are prevalent in the population could be used in recipients that have an MHC alloantigen together with the specific MHC type. The striking disconnect between the in vitro and in vivo results reported by Melenhorst et al demonstrates that caution is needed when in vitro results are interpreted for their possible significance in vivo.10 Further work, however, is needed to refine our understanding of the rules that govern the ability of alloreactive T cells to cause GVHD, because the results of a single study cannot dismiss all concerns about TCR cross-reactions. More sophisticated in vitro tests are needed to ensure that the results accurately reveal the true in vivo reality.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■