Abstract
Human volunteers receiving TGN1412, a humanized CD28-specific monoclonal antibody, experienced a life-threatening cytokine release syndrome during a recent trial. Preclinical tests using human PBMCs had failed to announce the rapid release of TNF, IFN-γ, and other toxic cytokines in response to this CD28 “superagonist” (CD28SA). CD28SA activate T-lymphocytes by ligating CD28 without overt engagement of the TCR. They do, however, depend on “tonic” TCR signals, which they amplify. Here we show that short-term preculture of PBMCs at high, but not at low, cell density results in massive cytokine release during subsequent stimulation with soluble TGN1412. Restoration of reactivity was cell-contact dependent, involved functional maturation of both monocytes and T cells, was sensitive to blockade by HLA-specific mAb, and was associated with TCR polarization and tyrosine phosphorylation. CD4 effector memory T cells were identified as the main source of proinflammatory cytokines. Importantly, responses to other T-cell activating agents, including microbial antigens, were also enhanced if PBMCs were first allowed to interact under tissue-like conditions. We provide a protocol, which strongly improves reactivity of circulating T cells to soluble stimulants, thereby allowing for more reliable preclinical testing of both activating and inhibitory immunomodulatory drugs.
Introduction
Immunomodulatory monoclonal antibodies (mAb) are a success story in the treatment of inflammation, autoimmunity, allograft rejection, and tumors. Their high biologic efficacy is, however, often accompanied by a severe but transient burst of systemic cytokine release, also known as a “cytokine storm.” For example, the first mAb to be applied to humans, the mAb OKT3, which binds to the CD3ϵ chain of the T-cell antigen-receptor (TCR) complex, induces high levels of circulating TNF, IFN-γ, and IL-2, 3 key pro-inflammatory mediators causing, inter alia, capillary leakage, leukocyte sequestration, and flu-like symptoms.1 In clinical practice, these undesired side effects are controlled by high-dose corticosteroids, which are routinely applied as premedication before the first administration of the mAb.2 Whereas in the case of OKT3, T cells are the obvious source of cytokine release, other therapeutic mAb, such as CAMPATH-1 or rituximab, which have no intrinsic T cell-activating potential, can also cause clinically relevant cytokine release, most likely through the ligation of Fc-receptors on other cytokine producers, such as NK cells.3,4
CD28 is a key costimulatory molecule expressed by virtually all CD4 but only about half of human CD8 T cells. Its ligands CD80 and CD86 are expressed on “professional” antigen-presenting cells, in particular on dendritic cells stimulated by microbial products through their innate receptors. Together with the antigenic peptide/MHC complexes, which are recognized by the TCR, CD80/86 costimulate T cells to proliferate and secrete cytokines.5
CD28 is a potential target for both inhibition and stimulation of T-cell responses. Ligand blockade with recombinant CTLA4-Ig has entered the clinic,6 and blockade of CD28 itself by mAb or mAb fragments has been successful in rodent and primate models.7-9 With regard to T-cell stimulation, mAb have been developed that activate T cells without the need to ligate the TCR, making them polyclonal T-cell activators.10-12 In contrast to conventional, costimulatory mAb, such CD28 “superagonists” (CD28SA) bind bivalently to laterally exposed determinants of CD28, allowing FcR-independent lattice formation.12-14 Importantly, the stimulatory activity of CD28SA depends on the presence of an intact TCR signaling machinery delivering weak or “tonic” signals,15,16 which are amplified by CD28 ligated by CD28SA at the level of the SLP76/Vav1/itk signalosome.15 In rats, CD28SA have the capacity to accelerate recovery from T-lymphopenia after irradiation and bone marrow reconstitution,17 whereas in immunocompetent rodents, a powerful induction of regulatory T cells was observed.18,19 The latter property has been exploited in a plethora of rat and mouse models of autoimmunity, inflammation, and transplantation, with extremely encouraging results.20-31
It was therefore a shock when TGN1412, a human CD28SA of the IgG4 subclass, caused a life-threatening cytokine release syndrome (CRS) during a first-in-man trial in March 2006.32 This tragic outcome was unexpected not only because of the benign behavior of CD28SA in analogous rodent models, but also because TGN1412 itself was well tolerated in cynomolgous monkeys.33 In the meantime, it is understood why these 2 datasets were not predictive for the human response: in rodents, Treg cells, fueled by IL-2 produced by conventional T cells, immediately quench the cytokine release19,34 ; their removal before CD28SA application leads to significant systemic cytokine levels.19 Most likely, this mechanism failed to protect the human volunteers because of the very different prevalence of the main source of proinflammatory cytokines in adult humans versus young laboratory mice: CD4 effector memory cells, which accumulate as a result of multiple exposure to infections and are the main source of toxic cytokines in response to TGN1412,4 are prominent in the human immune system but scarce in clean laboratory rodents. Accordingly, Treg cells in mice and rats, but not in humans, can control the triggering of cytokine production by these polyclonal T-cell activators. Recently, the failure of preclinical macaque testing to announce a CRS has also found a straightforward explanation: their failure to respond to TGN1412 with systemic cytokine release has been traced back to a lack of CD28 expression by CD4 effector memory cells in these primate species.4
Finally, preclinical testing with human PBMCs also did not warn of the substantial toxic potential of TGN1412.33 Thus, unlike the classic TCR agonist OKT3, which induces cytokine release both in vitro and in vivo, TGN1412 is inactive in vitro unless artificially immobilized on the plastic surface,35 a situation not reflecting in vivo interaction of the mAb with its target. In the present communication, we report that short-term culture at high cell density renders human PBMCs fully reactive to soluble TGN1412 in terms of cytokine release and cellular proliferation. We provide evidence supporting a model where the loss of weak or “tonic” TCR signaling, which results from cellular interactions in tissues, including scanning of MHC molecules,36 leads to unresponsiveness of circulating T cells, and where cellular interactions in high cell density cultures restore T-cell reactivity to the CD28SA.
Methods
PBMCs
Human PBMCs were prepared from healthy donors as a byproduct of platelet concentrates obtained with leukoreduction system chambers (LRS-C; Gambro Trima Accel aphaeresis apparatus, Pall Corp)37 and diluted in versene, or alternatively directly from heparinized venous blood, by density gradient centrifugation with Lymphocyte Separation Medium (PAA Laboratories) and washed with ice-cold balanced salt solution (BSS)/0.2% BSA.
Human lymph node cells
Lymph nodes were collected from the pancreas of nondiabetic brain-dead multiorgan donors received at the Islet Isolation Facility of the San Raffaele Hospital, Milan, Italy (Figure 5A). During the pancreas cleaning procedure and before islet processing, lymph nodes were removed from the fat and stromal tissues, immediately dipped in cold Belzer UW solution (Bristol-Myers Squibb), and kept on ice until processing (< 1 hour). Cells were kept in low cell density (1 × 106 cells/mL) for 1 hour, either on ice or in a humidified incubator at 37°C, 5% CO2.
Alternatively, lymph nodes were collected from the para-iliac region of renal transplant recipients at the Academic Medical Center, Amsterdam, The Netherlands (Figure 5B). Paired peripheral blood samples were collected before the transplantation procedure. Cells were frozen in IMDM supplemented with 10% DMSO, 20% FCS, penicillin, streptomycin, and 0.00036% β-mercaptoethanol.
The study was approved by the local ethics committee at the San Raffaele Diabetes Research Institute and the Academic Medical Center at the University of Amsterdam.
Lymph node dissociation experiment
Lymph node cells (1 × 106 cells/mL AB medium) were either stimulated immediately or placed in a humidified incubator (37°C, 5% CO2) for 2 or 4 hours, in a 50-mL Falcon tube with the lid off to allow gas exchange, shaking gently every 20 minutes to keep cells in suspension (Figure 5B).
Cell culture and stimulation assays
Cells were cultured in AB medium-RPMI 1640 supplemented with L-glutamine (Invitrogen), nonessential amino acids (Invitrogen), HEPES (Aplichem), β-mercaptoethanol (Invitrogen), sodium pyruvate (Invitrogen), penicillin/streptomycin, and 10% autologous or AB-positive heat-inactivated human serum (Sigma-Aldrich) in triplicates, using 96-well flat-bottom cell culture plates (Greiner Bio-One; 2 × 105 cells in 200 μL per well) in a humidified incubator at 37°C with 5% CO2. In the optimized “RESTORE” protocol, preculture was performed at 1.5 × 107 cells in 1.5 mL per well in 24-well suspension culture plates (Greiner) for 2 days. Cells were harvested with ice-cold AB medium and prepared for stimulation as described for fresh cells. GMP-grade TGN1412 was provided by TheraMab GmbH. Clinical grade OKT3 (Janssen-Cilag) was used as positive control. A mouse IgG2a isotype control was without effect, and the presence of approximately 100 μg/mL of IgG4 provided with 10% human serum served as isotype control for TGN1412.
For HLA blockade experiments, Fab fragments from anti–HLA class I (clone W6/32) and class II (clone Tü39), the kind gift of Dr Rammensee (Tübingen, Germany), were prepared using the Pierce Fab Preparation Kit (Thermo Scientific), and used at a final concentration of 10 μg/mL.
For depletion of cell subsets from PBMCs, cells were labeled with a primary antibody, followed by anti–FITC, anti–PE, or goat anti–mouse IgG microbeads (MACS), and depleted using MACS Columns and MACS Separator (Miltenyi Biotec). For isolation of cell subsets, Monocyte isolation kit II, human and CD4 T cell isolation kit II, human (Miltenyi Biotec) were used. Quality of depletion was confirmed by FACS analyses (data not shown).
Stimulation with pharmacologic inhibitors, immunomodulating agents, and antigens
Dexamethasone (Sigma-Aldrich), tetanus/diphtheria toxoid (Td; Sanofi-Pasteur), and PP1 (Merck Biosciences) were added as indicated.
Cell proliferation assays
Cell proliferation was measured from day 2 to 3 as radioactivity incorporated from [3H]thymidine (1 μCi per well; Hartmann Analytic GmbH) into DNA, using a Liquid Scintillation Counter (PerkinElmer Life and Analytical Sciences). Results are expressed as counts per minute (cpm). Alternatively, intracellular Ki67 staining was used to measure cell proliferation.
Analysis of cytokine concentration
Cell culture supernatants were analyzed for the presence of cytokines (TNF, IFN-γ, and IL-2) by Cytometric Bead Array (BD Biosciences), using an LSR II flow cytometer (BD Biosciences) following the manufacturer's instructions. Results were analyzed using FCAP Array Version 2.0 software (Soft Flow).
Antibodies and flow cytometry
The following anti–human antibodies were used: (1) CD4-PeCy5, Ki67-PE, CD45-Alexa647, all from Biolegend; (2) phosphotyrosine (pTyr, 4G10)–FITC, and unconjugated, both from Millipore; (3) CD45RO-allophycocyanin (APC), and FITC, TNF-PE, IFN-γ–PE, CD69-PE, CD14-PE, CCR7-Alexa647 and FITC, CD28-PECy5, CD56-PE, CD8-FITC, FoxP3-Alexa488, CD80-FITC, CD86-FITC, HLA-ABC–FITC, HLA-DR–FITC, HLA-DQ–FITC, and CD3-Alexa647, all from BD Biosciences. Secondary antibody anti–mouse Alexa488 was obtained from Invitrogen. Appropriate isotype controls were purchased from each company.
For phenotypic analysis, cells were stained with the appropriate antibodies for 15 minutes at 4°C, washed once with FACS buffer (PBS, 0.1% BSA, 2% sodium azide [NaN3]), and fixed with 2% paraformaldehyde. To analyze intracellular cytokine production, cells were stimulated, and protein secretion was blocked with brefeldin A (5 μg/mL, Sigma-Aldrich) during the last 4 hours of stimulation. After incubation, cells were stained for surface markers, permeabilized (Fix/Perm, eBioscience), and stained for intracellular TNF and IFN-γ, using Perm/wash (BD Biosciences). For intracellular staining of FoxP3 and Ki67, cells were first surface stained, permeabilized with Fix/Perm (eBioscience), and stained with the appropriate antibodies diluted in Perm/Wash (eBioscience). FACS analysis was performed using a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed using FlowJo Version 8.8.7 software (TreeStar). Results are shown as log10 fluorescence intensities.
Confocal analysis of human PBMCs and lymph nodes
Paraffin-embedded human lymph node sections (3 μm) were deparaffinized, boiled in citrate buffer (0.1M citric acid, 0.1M sodium citrate, pH 6.0), and blocked with PBS/10% BSA. Sections were stained as indicated.
PBMCs in ice-cold PBS/0.02% NaN3 were allowed to adhere to poly-lysine-coated glass slides, fixed with paraformaldehyde, and permeabilized with 0.1% Triton X-100 for 5 minutes, then stained as indicated. Stained PBMCs and lymph node sections were analyzed by confocal laser scanning microscopy with a Laser Scan Microscope, LSM510 Meta software (Version 3.2SP) equipped with inverted Zeiss Axiovert 200M stand and a 63×/1.4 numeric aperture objective lens (Zeiss).
When indicated, colocalization coefficients were determined using a Pearson algorithm, which ranged from −1 to 1, with values below 0.5 defined as low, between 0.5 and 0.65 as intermediate, and > 0.65 as high level of colocalization. More than 100 cells were counted per sample. Colocalization coefficients > 0.5 were considered significant. The pseudo-colored scatter plots shown display frequencies of the red-green pixels of the original images.
Statistical analysis
To evaluate statistically significant differences, the Wilcoxon signed rank-sum test, an unpaired t test, and a 2-way ANOVA were used. For correlation analysis, the Spearman rank correlation coefficient was performed. In all cases, a P value < .05 was considered as significant. Analyses were made using Prism Version 4.0c for Macintosh (GraphPad Software).
Results
Effect of preculture on PBMC reactivity to soluble TGN1412
We initially studied the in vitro response of freshly prepared human PBMCs to soluble TGN1412. As a positive control, the CD3 specific mAb OKT3 was used. Both reagents were added at 1 μg/mL, which is close to the estimated concentration of circulating TGN1412 during the London trial.38
After 24 hours, supernatants were analyzed for TNF, IFN-γ, and IL-2, which had been highly elevated in the plasma of the volunteers.32 Figure 1A shows that OKT3 efficiently induces cytokine release in these PBMC cultures, whereas, in agreement with published data,33,35 TGN1412 fails to do so.
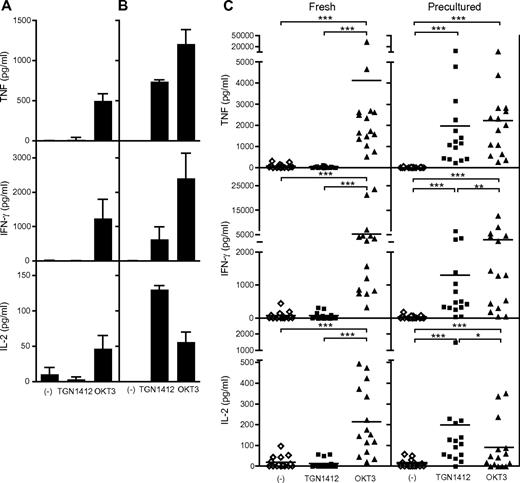
Induction of reactivity to the CD28 superagonist TGN1412 in human PBMCs by preculture. (A) Fresh PBMCs were cultured in 0.2 mL AB medium for 24 hours at 1 × 106 cells/mL in 96-well flat-bottom tissue culture plates. Cytokines in the supernatants were analyzed after 24 hours. mAb were used at 1 μg/mL. Isotype controls were negative (not shown). (B) PBMCs from the same donor as in Figure 1A were cultured in 1.5 mL AB medium for 2 days at 1 × 107 cells/mL in 24-well flat-bottom suspension culture plates before being washed and readjusted to 1 × 106 cells/mL. With these cells, the same experiment was performed as shown in Figure 1A. (C) Compiled data from 22 individual healthy donors. Conditions for antibody stimulation and for preculture were as in Figure 1A. Wilcoxon signed rank test: *P ≤ .05; **P ≤ .005; ***P ≤ .0001. − represents unstimulated cells. Data are mean ± SD of triplicate samples.
Induction of reactivity to the CD28 superagonist TGN1412 in human PBMCs by preculture. (A) Fresh PBMCs were cultured in 0.2 mL AB medium for 24 hours at 1 × 106 cells/mL in 96-well flat-bottom tissue culture plates. Cytokines in the supernatants were analyzed after 24 hours. mAb were used at 1 μg/mL. Isotype controls were negative (not shown). (B) PBMCs from the same donor as in Figure 1A were cultured in 1.5 mL AB medium for 2 days at 1 × 107 cells/mL in 24-well flat-bottom suspension culture plates before being washed and readjusted to 1 × 106 cells/mL. With these cells, the same experiment was performed as shown in Figure 1A. (C) Compiled data from 22 individual healthy donors. Conditions for antibody stimulation and for preculture were as in Figure 1A. Wilcoxon signed rank test: *P ≤ .05; **P ≤ .005; ***P ≤ .0001. − represents unstimulated cells. Data are mean ± SD of triplicate samples.
In one instance, PBMCs were “parked” in culture medium without stimulation for 2 days at 37°C. When these cells were used for the same experiment, TGN1412 unexpectedly induced a cytokine release of comparable magnitude as OKT3 (Figure 1B).
To evaluate the reproducibility of this phenomenon, PBMCs from 22 individual healthy donors were tested when freshly prepared, and again after 2 days of preculture. TGN1412 reactivity was consistently absent from fresh but present in precultured cells (Figure 1C). Thus, similar to the in vivo situation,2 both OKT3 and TGN1412 induce an “in vitro cytokine storm” if PBMCs are precultured before the assay is performed; in contrast, fresh PBMCs fail to reproduce the TGN1412 response observed during the clinical trial.
Characteristics of PBMC response to soluble TGN1412
Triggering of cytokine release by TGN1412 was dose-dependent and observed already at 0.06 μg/mL (Figure 2A), which translates into ∼ 5% receptor occupancy.38 At 1 μg/mL, the estimated plasma concentration during the London trial, which is now known to saturate 45% to 80% of CD28 molecules,38 the maximum biologic response was achieved. With regard to cellular source, production of TNF and IFN-γ in response to TGN1412 was restricted to CD4 memory cells (CD45RO+; Figure 2B). Indeed, analysis of 15 donors for the frequency of this subset and TNF release revealed a significant positive correlation (Spearman r = 0.70, P = .0039). Further subset analysis showed that, in keeping with recently published work using plastic-immobilized TGN1412,4 CD4 effector memory cells (CD45RO+CCR7−) are the main cytokine source (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Besides inducing cytokine release with a similar efficiency as OKT3, TGN1412 has comparable mitogenic activity if tested on precultured PBMCs, which again is predominantly seen in CD4+CD45RO+ cells (Figure 2C-D). TGN1412 reactivity is barely detectable if stimulation is performed after 1 day of preculture but is consistently observed if 2 days of preculture are used (Figure 2E; and data not shown).
Properties of T-cell responses induced by TGN1412 in high-density precultured PBMCs. (A) Precultured cells were stimulated with various mAb concentrations, and TNF was determined in supernatants after 24 hours. (B) Intracellular cytokine staining of high-density precultured PBMCs after stimulation with 1 μg/mL TGN1412 or OKT3. For optimal resolution, a responder with a particularly high TNF response was chosen. (C) Fresh and precultured PBMCs were prepared and cultured as described in legends to Figure 1A and B, respectively. Proliferation was determined by [3H]thymidine incorporation between days 2 and 3. (D) Proliferation of high-density precultured PBMCs by Ki67 staining, after 3-day stimulation with 1 μg/mL TGN1412 or OKT3. A different donor than in panel B is shown. (E) Fresh PBMCs were either stimulated immediately or placed in high density for 24 or 48 hours. They were then stimulated under standard conditions, and proliferation was determined by [3H]thymidine incorporation between days 2 and 3. (F) TNF release after TGN1412 stimulation of fresh and precultured PBMCs mixed at different ratios during the stimulation assay. Data are mean ± SD of triplicate samples.
Properties of T-cell responses induced by TGN1412 in high-density precultured PBMCs. (A) Precultured cells were stimulated with various mAb concentrations, and TNF was determined in supernatants after 24 hours. (B) Intracellular cytokine staining of high-density precultured PBMCs after stimulation with 1 μg/mL TGN1412 or OKT3. For optimal resolution, a responder with a particularly high TNF response was chosen. (C) Fresh and precultured PBMCs were prepared and cultured as described in legends to Figure 1A and B, respectively. Proliferation was determined by [3H]thymidine incorporation between days 2 and 3. (D) Proliferation of high-density precultured PBMCs by Ki67 staining, after 3-day stimulation with 1 μg/mL TGN1412 or OKT3. A different donor than in panel B is shown. (E) Fresh PBMCs were either stimulated immediately or placed in high density for 24 or 48 hours. They were then stimulated under standard conditions, and proliferation was determined by [3H]thymidine incorporation between days 2 and 3. (F) TNF release after TGN1412 stimulation of fresh and precultured PBMCs mixed at different ratios during the stimulation assay. Data are mean ± SD of triplicate samples.
Conditions of preculture required for acquisition of TGN1412 sensitivity
The acquisition of TGN1412 reactivity during preculture could be the result of a loss of suppression (eg, by preferential death of regulatory T cells), preferential survival of the TGN1412 responsive CD4 memory cell subset, differentiation of DC from monocyte precursors providing improved APC activity, or a gain of function in the responding T cells. Although preculture was associated with a modest loss of recovered viable cells (average recovery 67%), the subset composition of precultured PBMCs, and specifically that of regulatory and memory CD4 T cells as well as the minute representation of DC, remained stable compared with fresh PBMCs, arguing against the former possibilities (supplemental Figure 2). Nevertheless, we directly tested for a suppressive activity in fresh PBMCs by mixing them during the assay at different ratios with precultured PBMCs from the same donor (Figure 2F). TNF release obtained after TGN1412 stimulation was directly proportional to the fraction of precultured cells, suggesting that gain of function, rather than loss of suppression, explains the acquisition of TGN1412 reactivity by T cells during preculture.
Because “parking” of cells had been performed at a 10-fold higher cell density than is used for conventional PBMC assays, we tested the effect of this parameter on functional maturation. Preculture at high (107 cells/mL or 2 × 106 cells/cm2), but not at low density (106 cells/mL or 2 × 105 cells/cm2) led to TGN1412 responsiveness during subsequent stimulation (Figure 3A), suggesting a need for cell-cell contact. This was confirmed in a transwell system where PBMCs cultured at low density in the presence of a high-density culture separated by a semipermeable membrane failed to acquire TGN1412 reactivity (Figure 3A). Based on these observations, optimized conditions for restoring TGN1412 reactivity in PBMC cultures (the “RESTORE” protocol, for RESetting T cells to Original REactivity) were used in all subsequent experiments (“Cell culture and stimulation assays”).
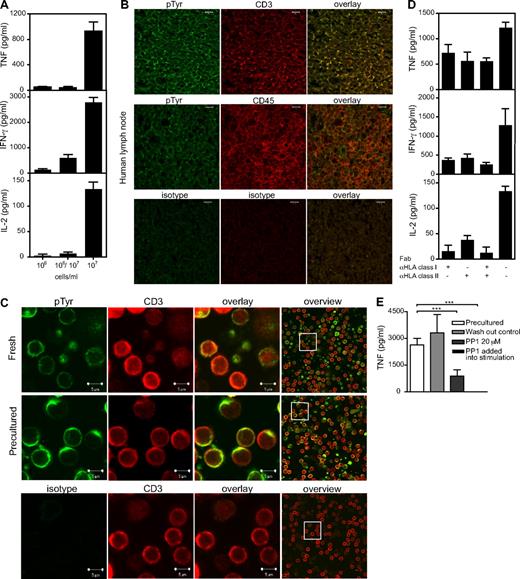
Role of cell-cell interaction and HLA recognition in acquisition of TGN1412 reactivity during high-density culture. (A) Cytokine release from cells precultured at high (107 cells/mL or 2 × 106 cells/cm2) or at low (106 cells/mL or 2 × 105 cells/cm2) density for 2 days, or at low density with a high-density transwell insert (106/107), and restimulated with 1 μg/mL TGN1412 for 24 hours. (B) Colocalization of CD3 with tyrosine-phosphorylated proteins in situ. Human lymph node sections were costained with anti–CD3 mAb (red) and anti–pTyr (green). Anti–CD45 mAb was included as a negative control. Appropriate isotype controls were used. (C) Fresh and high-density PBMCs were stained with anti–CD3 mAb (red) and anti–pTyr (green). An isotype control for pTyr was included. For statistical analysis of colocalization, see supplemental Figure 3. (D) High-density preculture of PBMCs was performed as described in Figure 1B. Fab fragments of mAb to HLA class I (W6/32) or HLA class II (Tü39) were added at 10 μg/mL. Secondary cultures were performed as in Figure 1. (E) The presence of Lck inhibitor PP1 throughout preculture affects TGN1412 response in secondary culture but is without effect when added as a short pulse before stimulation (washout control), and it fully blocks the response when added at the onset of the 24-hour stimulation. Two-way ANOVA: ***P ≤ .0001. Data are mean ± SD of triplicate samples.
Role of cell-cell interaction and HLA recognition in acquisition of TGN1412 reactivity during high-density culture. (A) Cytokine release from cells precultured at high (107 cells/mL or 2 × 106 cells/cm2) or at low (106 cells/mL or 2 × 105 cells/cm2) density for 2 days, or at low density with a high-density transwell insert (106/107), and restimulated with 1 μg/mL TGN1412 for 24 hours. (B) Colocalization of CD3 with tyrosine-phosphorylated proteins in situ. Human lymph node sections were costained with anti–CD3 mAb (red) and anti–pTyr (green). Anti–CD45 mAb was included as a negative control. Appropriate isotype controls were used. (C) Fresh and high-density PBMCs were stained with anti–CD3 mAb (red) and anti–pTyr (green). An isotype control for pTyr was included. For statistical analysis of colocalization, see supplemental Figure 3. (D) High-density preculture of PBMCs was performed as described in Figure 1B. Fab fragments of mAb to HLA class I (W6/32) or HLA class II (Tü39) were added at 10 μg/mL. Secondary cultures were performed as in Figure 1. (E) The presence of Lck inhibitor PP1 throughout preculture affects TGN1412 response in secondary culture but is without effect when added as a short pulse before stimulation (washout control), and it fully blocks the response when added at the onset of the 24-hour stimulation. Two-way ANOVA: ***P ≤ .0001. Data are mean ± SD of triplicate samples.
Characterization of cellular interactions leading to acquisition of TGN1412 reactivity during preculture
In search for an explanation for these surprising findings, the following previous observations were taken into consideration: (1) to activate T cells, CD28SA require weak or “tonic” TCR signals, which they amplify15,16 ; and (2) the TCR signaling machinery of mouse CD4 T cells residing in lymph nodes is “primed” by cellular adhesion,39 and by scanning MHC molecules on neighboring cells, resulting in its preassembly and facilitated signal transduction on antigen encounter.40 Importantly, mouse CD4 T cells lose this primed state when entering the circulation.40 We therefore hypothesized that PBMC-derived T cells had lost their primed status, which was recovered during preculture at high cell density by resuming cellular interactions and HLA scanning, providing a basal signal for amplification by TGN1412.
Because tyrosine phosphorylation is a key feature in the assembly of signaling complexes, we first compared the colocalization of the TCR/CD3 with tyrosine-phosphorylated proteins in human lymph node sections, and in fresh and high-density precultured PBMCs. In the lymph node, CD3 is expressed in a polarized fashion and colocalizes with phosphotyrosine staining, whereas CD45 staining does not (Figure 3B). In contrast, CD3 is homogeneously distributed at the surface of freshly isolated T cells, which are conspicuously low in tyrosine-phosphorylated proteins compared with CD3-negative cells (Figure 3C). Strikingly, phosphotyrosine expression is regained after 2 days of precultivation, where it appears in multiple cap-like structures, which costain with CD3-specific mAb (Figure 3C; for statistical evaluation of colocalization, see supplemental Figure 3).
To test directly whether the acquisition of TGN1412 reactivity during preculture is related to TCR scanning of HLA molecules, mAb reactive with all HLA class I and class II molecules were included during preculture and efficiently prevented acquisition of the TGN1412 response in the secondary cultures (not shown). Because intact mAb may lead to artifactual results because of negative signaling by cross-linked HLA molecules or FcR-mediated cytotoxicity, we also prepared monovalent Fab fragments for blocking studies. Again, we observed a strong reduction in the acquisition of TGN1412 reactivity (Figure 3D). Given that most of the cytokines produced in response to TGN1412 are released by CD4 T cells, it is counterintuitive that HLA I blockade should have an effect in this system. However, MHC scanning by the TCR is mediated by motifs built into the TCR V-segments and is independent of MHC class or allele.36,41 Blocking the Src-kinase Lck further tested the hypothesis that TCR signaling is required for functional maturation of circulating T cells. Inclusion of the inhibitor PP1 during preculture strongly reduced subsequent reactivity to TGN1412, whereas a brief pulse before harvest was without effect (washout control; Figure 3E). As expected from the known Lck dependence of the CD28SA response itself,42 PP1 fully blocked reactivity to TGN1412 when included during stimulation of precultured cells. Taken together, these results support the hypothesis that cellular interactions during high-density culture, including scanning of HLA molecules, promote the formation of signaling platforms that facilitate subsequent CD28SA reactivity.
In the lymphoid organs of mice, basal T-cell reactivity is mainly provided by scanning the surface of dendritic cells.43 By depleting individual PBMC populations, we found that restoration of T-cell responsiveness by preculture depends on the predominant population of antigen-presenting cells in PBMCs (ie, the monocytes) rather than on the very few dendritic cells found in the circulation (Figure 4A). To exclude that the observed effect was because of a requirement for monocytes during the assay for TGN1412 reactivity itself, we added PBMCs, which had been precultured at low cell density and thus contained all PBMC subsets but lacking functionally matured T cells. Such low-density precultured PBMCs were unable to restore T-cell reactivity during the TGN1412 assay, supporting our hypothesis that T-cell–monocyte interactions during the preculturing phase are required for acquisition of T-cell function.
Acquisition of TGN1412 reactivity by circulating T cells requires interaction with matured monocytes. (A) Before preculture at high density, PBMC subsets were depleted as indicated (−) by magnetic sorting (MACS). The group “All” refers to undepleted PBMCs. Cells were stimulated under standard conditions (Figure 1). In the lower panel, unseparated PBMCs cultured at low density (LDC) were added as a source of accessory cells at a 1:1 ratio. Proliferation was determined by [3H]thymidine incorporation between days 2 and 3. Representation of subsets before/after depletion was (%): B cells (5.79/0.014), monocytes (5.41/0.13), DC (0.68/0.0034), and MHC II+ (17.3/0.12). (B) Fresh (CFSE labeled) and high-density precultured PBMCs from the same donor were stimulated for 16 hours in low cell density, either separately or placed in coculture (1:1), with 1 μg/mL TGN1412 (blue) or OKT3 (red). Activation status was assessed by CD69 surface expression, gated on CD4+ cells. (C) Fresh CFSE-labeled PBMCs were added to 2-day high-density precultured PBMCs from the same donor and returned to high-density culture conditions for 0, 2, or 4 hours before stimulation under standard conditions with TGN1412 for 2 hours. Activation status was assessed by CD69 surface expression. Results are shown as percentage of CD69-positive cells among the CD4+CD45RO+ population. (D) Purified CD4+ T cells (5 × 105/0.5 mL) were cocultured in 48-well plates at the ratios given with monocytes derived from high-density PBMC cultures, or with monocytes isolated from fresh PBMCs and cultured for 2 days under high-density conditions, and stimulated with TGN1412 or OKT3 (1 μg/mL) for 16 hours. Activation status of CD4+ cells was assessed by CD69 surface expression. Gray histograms represent unstimulated cells. HDC indicates high-density cell cultures; LDC, low-density cell cultures; DC, dendritic cells; and MHC II+, major histocompatibility complex class II-positive cells. Unpaired t test: *P ≤ .05; **P ≤ .005. Data are mean ± SD of triplicate samples.
Acquisition of TGN1412 reactivity by circulating T cells requires interaction with matured monocytes. (A) Before preculture at high density, PBMC subsets were depleted as indicated (−) by magnetic sorting (MACS). The group “All” refers to undepleted PBMCs. Cells were stimulated under standard conditions (Figure 1). In the lower panel, unseparated PBMCs cultured at low density (LDC) were added as a source of accessory cells at a 1:1 ratio. Proliferation was determined by [3H]thymidine incorporation between days 2 and 3. Representation of subsets before/after depletion was (%): B cells (5.79/0.014), monocytes (5.41/0.13), DC (0.68/0.0034), and MHC II+ (17.3/0.12). (B) Fresh (CFSE labeled) and high-density precultured PBMCs from the same donor were stimulated for 16 hours in low cell density, either separately or placed in coculture (1:1), with 1 μg/mL TGN1412 (blue) or OKT3 (red). Activation status was assessed by CD69 surface expression, gated on CD4+ cells. (C) Fresh CFSE-labeled PBMCs were added to 2-day high-density precultured PBMCs from the same donor and returned to high-density culture conditions for 0, 2, or 4 hours before stimulation under standard conditions with TGN1412 for 2 hours. Activation status was assessed by CD69 surface expression. Results are shown as percentage of CD69-positive cells among the CD4+CD45RO+ population. (D) Purified CD4+ T cells (5 × 105/0.5 mL) were cocultured in 48-well plates at the ratios given with monocytes derived from high-density PBMC cultures, or with monocytes isolated from fresh PBMCs and cultured for 2 days under high-density conditions, and stimulated with TGN1412 or OKT3 (1 μg/mL) for 16 hours. Activation status of CD4+ cells was assessed by CD69 surface expression. Gray histograms represent unstimulated cells. HDC indicates high-density cell cultures; LDC, low-density cell cultures; DC, dendritic cells; and MHC II+, major histocompatibility complex class II-positive cells. Unpaired t test: *P ≤ .05; **P ≤ .005. Data are mean ± SD of triplicate samples.
Compared with the rapid functional recovery of murine APC-detached T cells during coculture with dendritic cells,43 the need to perform high-density cultures for 2 days to obtain optimal results with PBMCs (Figure 2E) suggested that monocytes are not immediately able to provide optimal interactions for promoting T-cell reactivity. We therefore tested whether monocytes derived from high-density PBMC cultures would allow T cells derived from fresh PBMCs to mount a more rapid response. As a readout allowing better temporal resolution, we chose the induction of the early activation marker CD69. Initially, we compared the TGN1412 response in fresh and high-density precultured PBMCs from the same donor and observed CD69 expression 16 hours later exclusively in CD4 T cells derived from the latter (Figure 4B). In addition, we labeled the fresh PBMCs with CFSE for later identification and cocultured them for stimulation at a 1:1 ratio with the precultured cells. In this setting, also fresh CD4 T cells responded, although to a much lower degree (Figure 4B). To follow acquisition of TGN1412 reactivity at higher temporal resolution, the mixed fresh and precultured PBMC cultures were left for another 0, 2, or 4 hours at high cell density before dilution and addition of TGN1412. CD69 expression was determined on CD4 memory cells (the most TGN1412-responsive subset) already 2 hours later (Figure 4C). Within the 4 hours of preincubation, the frequency of responding fresh cells doubled from around 5% to 10%, whereas those derived from the high-density precultured PBMCs remained stable at ∼ 20%. Together, these data indicate that the cellular environment of PBMCs precultured at high density permitted CD4 T cells from fresh PBMCs to rapidly acquire TGN1412 responsiveness, but not to the degree observed if the cells had been in continuous coculture at high density for 2 days.
We then tested directly whether coculture of monocytes with lymphocytes at high cell density had improved the functional status of monocytes, thereby allowing them to more rapidly promote reactivity to TGN1412. Monocytes were prepared either from fresh PBMCs and kept under high-density culture conditions for 2 days or they were isolated after high-density culture of the same PBMC preparation. Purified CD4 T cells from the same donor were cocultured with titrated numbers of these monocytes and stimulated with TGN1412 for 16 hours before determining CD69 expression (Figure 4D). Whereas monocytes derived from high-density PBMC cultures were highly effective “accessory cells,” those precultured at high cell density but in isolation were much less effective. Of note, the OKT3 response did not discriminate between the 2 monocyte preparations. Thus, the acquisition of optimal monocyte function required for induction of TGN1412 reactivity is, in turn, dependent on cellular interactions with lymphocytes during the preculturing step. Finally, we tried to identify possible phenotypic correlates for the improved monocyte function acquired during high-density cultures. Compared with fresh PBMC-derived monocytes, those present in high-density precultured PBMCs had up-regulated HLA class I and CD86, whereas only minor changes were observed for HLA class II and CD80 (supplemental Figure 4). Of note, the observed up-regulation depended on the presence of lymphocytes during preculture, supporting the hypothesis of a crosstalk between different types of PBMC-derived cells during high-density cell culture as a basis for the acquisition of a tissue-like functional status.
Response of lymph node T cells to TGN1412
The tragic outcome of the London TGN1412 trial indicates that, in contrast to circulating T cells, which constitute < 1% of all T cells in the human body, tissue-resident T cells, in particular of the CD4 effector memory type, which reside mostly in nonlymphoid tissues,44 do respond to TGN1412. We nevertheless used lymph node cells as an available source of tissue resident T cells because we predicted ex vivo responsiveness due to previous cellular interactions (Figure 5A). Single-cell suspensions were either left on ice or kept for 1 hour at 37°C to interrupt cellular contacts. A small, but significant proliferative response to TGN1412, as determined by thymidine incorporation, was observed, which dropped to approximately half when cells were preincubated in suspension at 37°C, thereby supporting the concept that loss of cellular interactions led to a loss of CD28SA reactivity. To identify the proliferating T-cell subset, TGN1412-stimulated lymph node cells were stained for surface markers and the nuclear proliferation marker Ki-67 (Figure 5B; supplemental Figure 5). Virtually all proliferative activity was observed within the fraction of CD4+CD45RO+ cells and declined again to approximately 30% and 25% if cells were dissociated for 2 or 4 hours, respectively (Figure 5B; supplemental Figure 5). Reactivity was not further improved by preculture of lymph node cells at high density (data not shown). Importantly, the CD4 memory cells present in fresh PBMCs from the same donor failed to respond (Figure 5B; supplemental Figure 5), in keeping with our earlier observations. Collectively, these results support our notion that cellular interactions within tissues enable CD4 memory T cells to respond to TGN1412.
Lymph node cells lose TGN1412 reactivity by suspension culture. (A) Human lymph node (LN) cells were kept on ice or at 37°C (5% CO2) for 1 hour in suspension (1 × 106 cell/mL), and the proliferative response to TGN1412 (1 μg/mL) was measured by [3H]thymidine incorporation between stimulation day 2 and 3. One of 2 individual experiments with similar results is shown. (B) Fresh LN cells were placed in suspension in low cell density (1 × 106 cell/mL), and either stimulated immediately (TGN1412 1 μg/mL) or kept in suspension culture at 37°C, 5% CO2, for 2 or 4 hours (top panel). A PBMC sample from the same donor was analyzed in parallel (bottom panel). Cell proliferation was determined after 4 days of stimulation by Ki67 staining, gated on CD4+ cells. One of 2 individual experiments with similar results is shown.
Lymph node cells lose TGN1412 reactivity by suspension culture. (A) Human lymph node (LN) cells were kept on ice or at 37°C (5% CO2) for 1 hour in suspension (1 × 106 cell/mL), and the proliferative response to TGN1412 (1 μg/mL) was measured by [3H]thymidine incorporation between stimulation day 2 and 3. One of 2 individual experiments with similar results is shown. (B) Fresh LN cells were placed in suspension in low cell density (1 × 106 cell/mL), and either stimulated immediately (TGN1412 1 μg/mL) or kept in suspension culture at 37°C, 5% CO2, for 2 or 4 hours (top panel). A PBMC sample from the same donor was analyzed in parallel (bottom panel). Cell proliferation was determined after 4 days of stimulation by Ki67 staining, gated on CD4+ cells. One of 2 individual experiments with similar results is shown.
Pharmacologic inhibition of T-cell responses after high-density preculture
Using RESTORE conditions, we were now able to ask questions about T-cell reactivity to TGN1412. First, we tested the ability of corticosteroids, the standard means for controlling the cytokine release syndrome frequently encountered during primary application of OKT3,2 to also control the TGN1412 response. In both cases, cytokine release was suppressed by dexamethasone, which prevents cytokine gene transcription, with the same efficiency (Figure 6A).
Studies with pharmacologic inhibitors and antigens. (A) Comparable sensitivity of cytokine release induced by OKT3 and TGN1412 to corticosteroid-mediated suppression. High-density precultured cells were prepared and stimulated as described in Figure 1B. Dexamethasone (Dex) was included at the final concentrations given, and cytokines were measured after 24 hours. (B) Fresh or precultured PBMCs (prepared as in Figure 1) were stimulated with a suboptimal concentration of OKT3 (0.01 μg/mL) in the presence of titrated amounts of PP1 as given. [3H]Thymidine incorporation was assessed between day 2 and 3. (C-E) Comparison of fresh and precultured PBMCs cultured as in Figure 1A and B. (C) Response to the bacterial superantigen SEB. (D) Overview over tetanus/diphtheria toxoid (Td) recall responses of 6 random donors tested either fresh or after 2-day high-density preculture. (E) Dose-response to Td of the strongest responder shown in panel D (blue line). Wilcoxon signed rank test: *P ≤ .05. Data are mean ± SD for triplicate samples.
Studies with pharmacologic inhibitors and antigens. (A) Comparable sensitivity of cytokine release induced by OKT3 and TGN1412 to corticosteroid-mediated suppression. High-density precultured cells were prepared and stimulated as described in Figure 1B. Dexamethasone (Dex) was included at the final concentrations given, and cytokines were measured after 24 hours. (B) Fresh or precultured PBMCs (prepared as in Figure 1) were stimulated with a suboptimal concentration of OKT3 (0.01 μg/mL) in the presence of titrated amounts of PP1 as given. [3H]Thymidine incorporation was assessed between day 2 and 3. (C-E) Comparison of fresh and precultured PBMCs cultured as in Figure 1A and B. (C) Response to the bacterial superantigen SEB. (D) Overview over tetanus/diphtheria toxoid (Td) recall responses of 6 random donors tested either fresh or after 2-day high-density preculture. (E) Dose-response to Td of the strongest responder shown in panel D (blue line). Wilcoxon signed rank test: *P ≤ .05. Data are mean ± SD for triplicate samples.
Second, we tested the effect of high-density preculture on T-cell responses elicited through the TCR. As shown in Figures 1 and 2, OKT3-induced T-cell proliferation and cytokine release do not require high-density preculture. Within the framework of our hypothesis, this is explained by the fact that the high-affinity ligand OKT3 addresses the TCR complex directly, obliterating the need for a primed TCR signaling machinery. Nevertheless, studies performed in mice show that TCR priming by MHC scanning enhances subsequent signaling intensity in response to cognate TCR ligands.40 Accordingly, we hypothesized that signal transduction in fresh PBMCs may be more sensitive to pharmacologic inhibition than in precultured PBMCs. This possibility was tested using OKT3 as stimulant and the Lck inhibitor PP1 to block signal transduction at the level of the earliest tyrosine phosphorylation steps during TCR-mediated signal transduction. As shown in Figure 6B, sensitivity to PP1 is indeed reduced by preculture, suggesting that the use of fresh PBMCs for screening and development of immunosuppressant drugs directed at T cells may be misleading because the target cells used are not fully reactive.
Effect of high-density preculture on responses to bacterial antigens
The quantitation of recall responses and the identification of antigen-specific memory T cells are routinely performed on freshly isolated PBMCs. However, mouse TCR-transgenic CD4 T cells lose sensitivity to their cognate antigen during recirculation.40 Accordingly, we asked whether the response to recall antigens would be improved by resetting PBMCs to tissue-like status during high-density preculture. Proliferative recall responses to a combined tetanus/diphtheria toxoid preparation and the response to the bacterial superantigen SEB were indeed dramatically enhanced by this procedure (Figure 6C-E).
Discussion
Here we report that, although freshly isolated PBMCs fail to respond to TGN1412, the CD28 superagonist, which caused a massive cytokine storm during a first in human trial in 2006, 2 days of preculture at a 10-fold higher cell density than is usually used for in vitro assays endows them with full responsiveness, which is comparable with the well-studied T-cell activating mAb OKT3 with its similar in vivo toxicity profile. Both monocytes and T cells up-regulate functional activity, possibly by acquiring tissue-like properties during high-density culture. Indeed, cell-cell contacts are a prerequisite for functional maturation, which probably involves both adhesion-mediated up-regulation of sensitivity to TCR signals, as previously described,39 and subthreshold priming of the TCR signaling machinery itself by scanning of cell surfaces for MHC molecules.40 Lack of such cellular interactions was previously found in mice to reduce T-cell responsiveness of circulating compared with tissue-resident T cells to cognate antigens.40 However, recovery of reactivity in the mouse system on coculture with dendritic cells occurs within hours rather than 2 days,43 a difference probably the result of an initial requirement for monocyte maturation in high-density PBMC cultures to subsequently allow subthreshold T-cell priming. Indeed, monocytes from high-density cultures are able to rapidly increase sensitivity of CD4 T cells to subsequent TGN1412 stimulation (Figure 4D).
The outcome of the TGN1412 trial indicates that, in contrast to circulating T cells, tissue resident CD4 effector memory cells immediately responded to the drug with cytokine release.32 We here propose that the failure of conventional PBMC assays to warn of the CRS was the result of the loss of subthreshold T-cell activation acquired during cellular interactions within the tissues. The reactivity of lymph node cells, as opposed to PBMCs, to mount a TGN1412 response (Figure 5; supplemental Figure 5) supports this hypothesis.
Because immune responses are initiated in lymphoid tissues, which enable cellular interactions, and effector functions also occur within tissues, the loss of such subthreshold signals is of no importance for the functionality of our immune system but has serious implications for PBMC-based assays as they are routinely performed in basic immunology, preclinical development, and diagnostic testing. Because for most T-cell stimulants, the proposed reduction in excitability resulting from interrupted cellular contacts is not absolute, the impaired responsiveness of circulating T cells has not been previously appreciated in humans. In mice, however, it is readily revealed that responsiveness to cognate MHC/peptide complexes is reduced by approximately 10-fold in circulating compared with tissue-resident T cells.40 Similarly, we find that recall responses as well as the response to the superantigen staphylococcal enterotoxin B are greatly increased in sensitivity if PBMCs are first allowed to interact under high-density culture conditions (Figure 6). Initially, however, the impairment of T-cell reactivity in PBMCs resulting from “separation from self” became obvious to us because of the stringent dependence of CD28SA-mediated T-cell activation on weak TCR signals.15,16 It was the all-or-nothing effect of the TGN1412 response to fresh versus precultured PBMCs, which prompted us to examine the mechanistic basis and the importance of TCR-priming for T-cell responses in general.
With regard to the substance TGN1412 itself, resetting PBMCs to tissue-like conditions allowed, for the first time, to reveal the T-cell response to soluble TGN1412 in vitro, to establish a relationship between receptor occupancy and cytokine release, and to test for the effectiveness of established drugs in controlling a TGN1412 triggered cytokine release syndrome. Thus, the threshold for TNF induction by TGN1412 is < 5% receptor occupancy, whereas the likely level achieved during the first-in-man trial was approximately 45% to 80%38 and in functional saturation (Figure 2A). These results support a MABEL-based approach to the calculation of an entry dose in phase 1 studies, and a discontinuation of NOAEL-based calculations, which provided a misleading result for the TGN1412 trial because of an unexpected absence of CD28 on CD4 effector memory cells in macaques.4,33 Furthermore, our in vitro data strongly suggest that release of proinflammatory cytokines by CD28SA stimulation is as sensitive to corticosteroid medication as is known for OKT3 (Figure 6A), which is expected based on the transcriptional repression of cytokine synthesis by activated glucocorticoid receptors.
Plausible explanations for the failure of rodent and macaque models to predict the TGN1412-induced CRS have been provided,4,19,45,46 but the failure of conventional PBMC assays to respond to the drug in its soluble form has remained unclear. Based on its lack of ADCC activity47 and the generally poor FcR binding ability of IgG4 in the presence of a huge access of competing high-affinity binders present in culture medium on the one side, and the presence of FcR-positive B cells and monocytes within fresh PBMC preparations on the other, insufficient FcR-mediated crosslinking in PBMC assays compared with lymphoid and other tissues seemed an unlikely explanation for the unresponsiveness of fresh PBMCs, a notion confirmed by the stimulatory activity of the TGN1412 Fab2 fragment (P.S.R., S.B., and T.H., unpublished observations). Our findings show that the CD28 crosslinker TGN1412 has a similar but not identical functional profile compared with the CD3-crosslinker OKT3 and suggest that, in contrast to the latter, it requires preceding cellular interactions to render T cells sensitive to the activating stimulus.
Beyond better understanding TGN1412, our findings have the general implication that resetting PBMCs to lymph node-like activity will improve the predictive value of T cell–directed preclinical assays in general (eg, by providing a better estimate of the effectiveness of antiphlogistic intervention during preclinical studies) and on an individualized or “personalized” level for each potential recipient.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank TheraMab for providing TGN1412, H.-G. Rammensee (Tübingen, Germany) for the kind gift of the mAb W6/32 and Tü39; A. Opitz (Clinical Transfusion Medicine, University of Würzburg) for providing peripheral blood samples, and E. Remmerswaal for her help with LN and PBMC paired samples.
This work was supported by Bayerisches Immuntherapie-Netzwerk and Deutsche Forschungsgemeinschaft through SFB581.
Authorship
Contribution: P.S.R. and S.B. prepared the PBMC samples, performed and analyzed the stimulation, stainings, and proliferation assays, and measured cytokines from supernatants; S.-Y.N. performed confocal analysis of human lymph node sections; E.A. performed confocal analysis of human PBMCs; M.B. obtained, stimulated, and measured proliferation of human lymph node cells; I.t.B. provided paired lymph node and PBMC samples; P.S.R. and T.H. designed the experiments and analyzed data; and T.H. designed and directed the study and wrote the report, with input from H.E. and P.S.R.
Conflict-of-interest disclosure: T.H. is a consultant to TheraMab. The remaining authors declare no competing financial interests.
Correspondence: Thomas Hünig, Institute of Virology and Immunobiology, University of Würzburg, Versbacher Strasse 7, 97078, Würzburg, Germany; e-mail: huenig@vim.uni-wuerzburg.de.

![Figure 2. Properties of T-cell responses induced by TGN1412 in high-density precultured PBMCs. (A) Precultured cells were stimulated with various mAb concentrations, and TNF was determined in supernatants after 24 hours. (B) Intracellular cytokine staining of high-density precultured PBMCs after stimulation with 1 μg/mL TGN1412 or OKT3. For optimal resolution, a responder with a particularly high TNF response was chosen. (C) Fresh and precultured PBMCs were prepared and cultured as described in legends to Figure 1A and B, respectively. Proliferation was determined by [3H]thymidine incorporation between days 2 and 3. (D) Proliferation of high-density precultured PBMCs by Ki67 staining, after 3-day stimulation with 1 μg/mL TGN1412 or OKT3. A different donor than in panel B is shown. (E) Fresh PBMCs were either stimulated immediately or placed in high density for 24 or 48 hours. They were then stimulated under standard conditions, and proliferation was determined by [3H]thymidine incorporation between days 2 and 3. (F) TNF release after TGN1412 stimulation of fresh and precultured PBMCs mixed at different ratios during the stimulation assay. Data are mean ± SD of triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/26/10.1182_blood-2010-12-319780/4/m_zh89991181560002.jpeg?Expires=1765887593&Signature=lehrM0Wa4lAUKacV-~y5ul12dRV5Wb8aB7dP8V8zxreYCBo0SZahQCPu2x4woHHODUvGSCpri~8E6xUW5F4kYkX0HVGyi2QDkPK~lRv-5jLnnCxCcDaPJdkaoHGrrptiNJKoV6HTtGzwPLXygvRn1JWAbZ6btBRDdFtIwKmg38nGSENjXrKKvh9~aB1b8sHbEAqnhpKoarBiCCr78xdEjjsbAIeUeBYxML~QeH-MPzZ-io9hIV3iWVMGNsyQk2wLSB1yaqM30frXTcTPkxAhNzy-1l9Sjau9zxq~9gCWe~hNN8kzJ1AQhmZBtH~4vlHJEfSBL1EUybEUY2RWzmK2Kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Acquisition of TGN1412 reactivity by circulating T cells requires interaction with matured monocytes. (A) Before preculture at high density, PBMC subsets were depleted as indicated (−) by magnetic sorting (MACS). The group “All” refers to undepleted PBMCs. Cells were stimulated under standard conditions (Figure 1). In the lower panel, unseparated PBMCs cultured at low density (LDC) were added as a source of accessory cells at a 1:1 ratio. Proliferation was determined by [3H]thymidine incorporation between days 2 and 3. Representation of subsets before/after depletion was (%): B cells (5.79/0.014), monocytes (5.41/0.13), DC (0.68/0.0034), and MHC II+ (17.3/0.12). (B) Fresh (CFSE labeled) and high-density precultured PBMCs from the same donor were stimulated for 16 hours in low cell density, either separately or placed in coculture (1:1), with 1 μg/mL TGN1412 (blue) or OKT3 (red). Activation status was assessed by CD69 surface expression, gated on CD4+ cells. (C) Fresh CFSE-labeled PBMCs were added to 2-day high-density precultured PBMCs from the same donor and returned to high-density culture conditions for 0, 2, or 4 hours before stimulation under standard conditions with TGN1412 for 2 hours. Activation status was assessed by CD69 surface expression. Results are shown as percentage of CD69-positive cells among the CD4+CD45RO+ population. (D) Purified CD4+ T cells (5 × 105/0.5 mL) were cocultured in 48-well plates at the ratios given with monocytes derived from high-density PBMC cultures, or with monocytes isolated from fresh PBMCs and cultured for 2 days under high-density conditions, and stimulated with TGN1412 or OKT3 (1 μg/mL) for 16 hours. Activation status of CD4+ cells was assessed by CD69 surface expression. Gray histograms represent unstimulated cells. HDC indicates high-density cell cultures; LDC, low-density cell cultures; DC, dendritic cells; and MHC II+, major histocompatibility complex class II-positive cells. Unpaired t test: *P ≤ .05; **P ≤ .005. Data are mean ± SD of triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/26/10.1182_blood-2010-12-319780/4/m_zh89991181560004.jpeg?Expires=1765887593&Signature=Sso0lz6jWelhrs1XaTPmMf28bwSnvbIOUoNCXNPwzOJZtAmfJiQSx6vy5bgFD9ziXejDNfjXxhjNvW1nn4cQuJwcvKBVyVNW5l2FYh-3IyxKYSKmH6UDFFKdI~bMRCSi7bHZmY~sj76Q-ghEZDXXTp3pn7KkTe-Bf61Zk-c46v7ynGKjnxCHXWr1tYHQbe3RHep1NgxIChJsI9pD7ueHln414PCOY41FBRUv3pKTCw~MygIJVUEfqf0bmMMiMcmAb~hjGukL5drYTZEmYKSaxTqPSe8DxAyIeyE9S4D8CCdvzkLZg8LLjll7pTkDrOMlFUs0tRLg~7zqUnjbGz4WGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Lymph node cells lose TGN1412 reactivity by suspension culture. (A) Human lymph node (LN) cells were kept on ice or at 37°C (5% CO2) for 1 hour in suspension (1 × 106 cell/mL), and the proliferative response to TGN1412 (1 μg/mL) was measured by [3H]thymidine incorporation between stimulation day 2 and 3. One of 2 individual experiments with similar results is shown. (B) Fresh LN cells were placed in suspension in low cell density (1 × 106 cell/mL), and either stimulated immediately (TGN1412 1 μg/mL) or kept in suspension culture at 37°C, 5% CO2, for 2 or 4 hours (top panel). A PBMC sample from the same donor was analyzed in parallel (bottom panel). Cell proliferation was determined after 4 days of stimulation by Ki67 staining, gated on CD4+ cells. One of 2 individual experiments with similar results is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/26/10.1182_blood-2010-12-319780/4/m_zh89991181560005.jpeg?Expires=1765887593&Signature=Z51B6HpUOzRgmClVNJPn99cnK95Vf6vn934BlzSnh2H7g9D8Oo64aP6-Efdzd9msI-uFBmhmGHRZ1OujoUj6dix3bXcHBygLqYxLgV32d7bahXYIT4bvSpSp1W4hNL6V-DIpBAZ2XI0GvB09yEVHnhhKh10~gizISWRcQ97uipMxPYUYyg~Cux0W2wBy2cmHEv4BlziDQBhB3uuX9C-MXsCyET1UWeHkrWz9FuxKMh8Cd28gQA4B9gAF2wTzNLs33fLhQnESFdIQygWJJFnXwToFPe9jP7LKxYuDuEAXjMDVWGyxhGOhWw9B6yb23ghdKwLg9~GOTlsRvXS1URrMpg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Studies with pharmacologic inhibitors and antigens. (A) Comparable sensitivity of cytokine release induced by OKT3 and TGN1412 to corticosteroid-mediated suppression. High-density precultured cells were prepared and stimulated as described in Figure 1B. Dexamethasone (Dex) was included at the final concentrations given, and cytokines were measured after 24 hours. (B) Fresh or precultured PBMCs (prepared as in Figure 1) were stimulated with a suboptimal concentration of OKT3 (0.01 μg/mL) in the presence of titrated amounts of PP1 as given. [3H]Thymidine incorporation was assessed between day 2 and 3. (C-E) Comparison of fresh and precultured PBMCs cultured as in Figure 1A and B. (C) Response to the bacterial superantigen SEB. (D) Overview over tetanus/diphtheria toxoid (Td) recall responses of 6 random donors tested either fresh or after 2-day high-density preculture. (E) Dose-response to Td of the strongest responder shown in panel D (blue line). Wilcoxon signed rank test: *P ≤ .05. Data are mean ± SD for triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/26/10.1182_blood-2010-12-319780/4/m_zh89991181560006.jpeg?Expires=1765887593&Signature=vgQd9f0XbLEyGaD7EMiUO~d4-rrIIYulagVzIgKuGE2t2ovZ3u4Xbq1Maywk2tE2mmNZQ-na~L6xDkclZnxAZtydNco~5QcIaDK27viGHAVvLYwSPCij9CTo3WGzQt68hntAYI2HZwutFD-YaYlrToHcKRmd-5GQLNv3aN4Jzd8EDntHKj23~C71IYOh6RWpzmlAn3t5dzOcFOZa6CAAv7-3K0XFQtz4R~Jn~kvoyykx7-rNTLeO1FlFFDvGI1fqb4-7TyiWouN4noiyETmbOtPSgslXqDw0h3BT9wZTyhnX-lJVnPn3tlISMQR1FEICZoQlSjHuCYtFIBPYlG7zSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)