Abstract
Many transformed lymphoma cells show immune-phenotypes resembling the corresponding normal lymphocytes; thus, they provide a guide for proper diagnosis and present promising routes to improve their pathophysiologic understanding and to identify novel therapeutic targets. However, the underlying molecular mechanism(s) of these aberrant immune-phenotypes is largely unknown. Here, we report that microRNA-135b (miR-135b) mediates nucleophosmin-anaplastic lymphoma kinase (NPM-ALK)–driven oncogenicity and empowers IL-17–producing immunophenotype in anaplastic large cell lymphoma (ALCL). NPM-ALK oncogene strongly promoted the expression of miR-135b and its host gene LEMD1 through activation of signal transducer and activator of transcription (STAT) 3. In turn, elevated miR-135b targeted FOXO1 in ALCL cells. miR-135b introduction also decreased chemosensitivity in Jurkat cells, suggesting its contribution to oncogenic activities of NPM-ALK. Interestingly, miR-135b suppressed T-helper (Th) 2 master regulators STAT6 and GATA3, and miR-135b blockade attenuated IL-17 production and paracrine inflammatory response by ALCL cells, indicating that miR-135b–mediated Th2 suppression may lead to the skewing to ALCL immunophenotype overlapping with Th17 cells. Furthermore, antisense-based miR-135b inhibition reduced tumor angiogenesis and growth in vivo, demonstrating significance of this “Th17 mimic” pathway as a therapeutic target. These results collectively illuminated unique contribution of oncogenic kinase-linked microRNA to tumorigenesis through modulation of tumor immune-phenotype and microenvironment.
Introduction
MicroRNAs (miRNAs) are endogenous noncoding, 20- to 23-nucleotide single-stranded RNAs that negatively regulate gene expression in a sequence-specific manner.1 miRNA species are generated through RNase-mediated processing reaction by two central RNases III, Drosha and Dicer, from long primary transcripts (primary miRNAs [pri-miRNAs]) and incorporated along with core Argonaute proteins into the RNA-induced silencing complex. RNA-induced silencing complex interacts mainly with 3′ untranslated region (UTR) of target mRNAs through partial base complementarity to the 5′ miRNA seed region, leading to degradation, destabilization, or translational inhibition of target mRNAs. miRNAs regulate differentiation and functions of various cell types, including immune cells in a highly context-dependent manner.2
Alteration of miRNome has emerged as a key feature of cancer-associated dysfunction of gene regulatory networks. Although miRNA dysregulation affects cancer cell behavior with other genetic and epigenetic abnormalities, full pictures of their causes and consequences remain to be elucidated.3 In hematologic malignancies, many transformed lymphoma cells show immune-phenotypes resembling the corresponding normal lymphocytes; thus, they represent a guide for proper diagnosis and promising routes to improve our understanding of their pathogenesis and to identify novel therapeutic targets.4 For example, diffuse large B-cell lymphomas have been shown to be composed of at least 2 prognostic entities, depending on its resemblance to normal germinal center or activated B cells.5 However, the molecular basis shaping the aberrant immunophenotypes of tumor cells has been largely unknown, and the relationship between miRNA regulation and lymphoma phenotype has not been investigated.
Recent studies revealed several mechanisms regulating miRNA expression.6 Although certain oncoproteins, including Myc and tumor suppressors such as p53, have been linked to the regulation of miRNA expression,7,8 involvement of oncogenic tyrosine kinases remains unclear in this regulatory pathway. Anaplastic lymphoma kinase (ALK) exerts characteristic oncogenic activities through fusion to several gene partners or mutations both in hematopoietic and nonhematopoietic solid tumors.9,10 Nucleophosmin (NPM)–ALK is a representative translocation-dependent fusion-type oncogenic tyrosine kinase in anaplastic large cell lymphoma (ALCL). Although NPM-ALK drives malignant transformation of ALCL cells through various molecular mechanisms, including activation of signal transducer and activator of transcription (STAT) 3, Ras-ERK, and PI3K oncogenic signaling pathways,9 involvement of miRNAs has not been reported so far. Here, we have explored unrecognized involvement of miRNAs in the downstream of NPM-ALK.
We identified miR-135b as one of the major downstream executors of NPM-ALK chimeric oncoprotein in ALCL. NPM-ALK strongly promoted the expression of miR-135b and its host gene LEMD1 through STAT3 activation. The elevated miR-135b targeted FOXO1 tumor suppressor. Interestingly, we further revealed the immune modulatory property of miR-135b shaping the T-cell phenotypes of ALCL cells. miR-135b suppressed T-helper (Th) 2 master regulators STAT6 and GATA3, and the blockade of miR-135b attenuated IL-17 production and paracrine inflammatory response by ALCL cells, suggesting that miR-135b–mediated Th2 suppression may skew the ALCL immunophenotype to overlap with that of Th17 cells. Antisense-based miR-135b inhibition reduced tumor angiogenesis and growth in vivo, underscoring the pathogenic roles of this pathway. Our findings revealed that miR-135b is involved in NPM-ALK–driven tumorigenesis and modulation of ALCL immunophenotype, and they also suggest dynamic commitment of miRNAs to mutual regulation between Th cell differentiation programs and determination of polarized immunophenotypes of malignant cells.
Methods
Cell lines and reagents
Karpas 299, SUDHL-1, and SUP-M2 cell lines were obtained from the German Collection of Microorganisms and Cell Cultures. Jurkat, Molt4, CCRF-CEM, HCT116, HEK293T, HeLa, and lung cancer cell lines were obtained from the American Type Culture Collection. WI-38 human diploid fibroblast line was obtained from the RIKEN Cell Bank. Human normal peripheral blood pan T lymphocytes were purchased from AllCells. Neuroblastoma cell lines were kindly provided from R. Sakai (National Cancer Center Research Institute, Tokyo, Japan). Hematologic, neuroblastoma, and lung cancer cell lines were maintained in RPMI-1640 (Invitrogen) containing 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. Other cell lines were maintained in Dulbecco modified eagle medium (Invitrogen) with 10% FBS. In coculture experiment, Karpas 299 and WI-38 cells were cocultured (in Opti-MEM [Invitrogen] with 1% FBS) for 48 hours using Transwell tissue culture inserts (0.4-μm pore size; BD Biosciences). The following antibodies were used: ALK 4C5B8 (Invitrogen); STAT3 124H6, p-STAT3 D3A7, Akt 9272, p-Akt 193H12, FOXO1 C29H4, and STAT6 9362 (Cell Signaling Technology); p21 H-164, p27 F-8, and GATA3 HG3-31 (Santa Cruz Biotechnology); CREG1 299133 (R&D Systems); CD31 555024 (BD Biosciences); and α-tubulin DM-1A (Sigma-Aldrich). Kinase inhibitors (WHI-P154, U0126 and LY294002) were purchased from Calbiochem.
Patient samples
ALCL patients were diagnosed at Juntendo University Hospital and Juntendo Urayasu Hospital. Investigations were carried out in accordance with ethical standards authorized by the ethics committees of University of Tokyo, Juntendo University School of Medicine, and Jichi Medical University. Written informed consent was obtained in accordance with the Declaration of Helsinki.
TuD miRNA system, shRNA, and plasmids
Tough decoy RNA (TuD RNA) against miR-135b was designed according to a previous report.11 Detailed structures of TuD RNA are described in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). shRNAs were designed as described previously.12,13 TuD RNAs and shRNAs were introduced into pENTR-H1 vector. Pri-miRNA expression vectors were generated by cloning short fragments of pri-miRNAs containing pre-miRNA and flanking sequence on both sides of pre-miRNA into pcDNA6.2-GW/EmGFP-miR (Invitrogen). miRNA sensor vectors were prepared by inserting mature miRNA complementary sequences within the XhoI and NotI sites of the 3′UTR of the luciferase gene in the Psicheck 2 dual luciferase reporter vector (Promega). For other reporter constructs, the 3′UTR segment of each target gene was cloned into the same luciferase reporter vector. The primer sequences used are given in supplemental Table 2. For transient transfection, pre-miR miRNA precursors (Ambion) also were used.
Luciferase reporter assay
Cells were transfected with each reporter construct with pri-miRNA expression vector using Lipofectamine 2000 (Invitrogen). Cell extracts were prepared 24 to 48 hours after transfection, and the ratio of Renilla to firefly luciferase was measured using the Dual-Luciferase Reporter Assay System (Promega).
qRT-PCR assays
Quantitative (q)RT-PCR assays were performed as described previously.8 For detection of mRNAs, total RNA was extracted by TRIzol (Invitrogen) and subjected to reverse transcription using the PrimeScriptII first-strand cDNA synthesis kit (Takara) according to the manufacturer's instructions. qRT-PCR was performed with the 7500 Fast Real-Time PCR System (Applied Biosystems). The expression levels of mature miRNAs were determined using TaqMan MicroRNA assay kit (Applied Biosystems) according to the manufacturer's protocol. Data analysis was done by the comparative CT method. Results were normalized to β-actin for pri-miRNA detection, and RNU44 small nucleolar RNA for evaluation of mature miRNA. miRNeasy mini kit (QIAGEN) or RecoverAll total nucleic acid isolation kit for FFPE Tissues (Ambion) was used for RNA extraction from clinical samples. The primer sequences used are given in supplemental Table 3.
Chromatin immunoprecipitation analysis
Cells were fixed by adding formaldehyde and then harvested. After sonication, samples were incubated at 4°C overnight with protein A or anti–mouse IgG-Dynabeads that had been preincubated with 5 to 10 μg of antibodies in PBS and 0.5% BSA. To precipitate STAT3, anti-STAT3 antibody 124H6 (Cell Signaling Technology) was used. Immunoprecipitated samples were eluted and reverse-crosslinked by incubation overnight at 65°C. Genomic DNA was then extracted with a PCR purification kit (QIAGEN) and subjected to PCR analysis. The primer sequences used are given in supplemental Table 3.
Lentiviral gene transfer
TuD RNAs, shRNAs, pri-miRNA, NPM-ALK, or mouse constitutively active (ca)–STAT3 (A662C/N664C)14 was introduced by lentiviral infection system (a kind gift from H. Miyoshi, RIKEN, Tsukuba, Japan). TuD RNAs and shRNAs were transferred into lentivirus vector CS-RfA-EG via pENTR-H1 vector using LR clonase. Pri-miRNA was similarly transferred into CSII-EF-RfA-CMV-Puro lentivirus vector using pENTR vector. The lentivirus production was carried out by transfection of HEK293FT cells with the vector construct pCMV-VSV-G-RSV-Rev and pCAG-HIVgp. The viral particles were collected 48 hours after transfection, titered by Lenti-X qRT-PCR titration kit (Takara), introduced to cultured cells, and monitored by flow cytometric analysis to determine infection efficiency.
Immunoblot assay
Cells were lysed with a buffer containing 1% Nonidet P-40, 20mM Tris-HCl, pH 7.4, 150mM NaCl, 5mM EDTA, and 1% protease inhibitor mixture (Nacalai Tesque). Total cell lysates were subjected to SDS-PAGE and transferred to Fluoro Trans W membrane (Pall). Immunoblotting was performed using the indicated antibodies.
Drug sensitivity assay
Jurkat cells were seeded at a density of 1 × 105/mL and split 24 hours before treatment. After 24-hour treatment with different concentrations of cytosine β-d-arabinofuranoside (Sigma-Aldrich), cells were collected, washed, and reseeded for assay of cell viability and apoptosis. Cell viability and apoptosis were assessed by WST-8 colorimetric assay (Nacalai Tesque) and annexin V assay kit (BD Biosciences), respectively.
ELISA
After transfection with miRCURY LNA microRNA power inhibitor (EXIQON; Control A or antisense against miR-135b), IL-17F concentrations in the culture supernatant (72 hours) of Karpas 299 cells were determined by ELISA kit (R&D Systems; Duoset human IL17F), according to the manufacturer's instructions.
In vivo cancer models
C.B-17/IcrCrj severe combined immunodeficient (SCID) female mice (4 weeks of age) were obtained from Charles River Japan. All animal experimental protocols were performed in accordance with the policies of the Animal Ethics Committee of the University of Tokyo. Karpas 299 cells (1 × 106; n = 6/group) were subcutaneously injected in 0.2 mL of a mixture of RPMI-1640 without FBS and 30% Matrigel (BD Biosciences) into female SCID mice and allowed to grow for 1 week to reach a volume of 50 to 200 mm3. Complexes of miRCURY LNA microRNA power inhibitor (EXIQON; Control A or antisense against miR-135b) and atelocollagen (Koken) were prepared according to the manufacturer's instructions. Antisense oligonucleotides (5μM) with atelocollagen in a 200-μL volume were administered into the subcutaneous spaces around the tumors at days 7 and 10 after inoculation. Subcutaneous xenografts were measured externally every day until the end of evaluation periods, and tumor volume was approximated using the equation vol = (a × b2)/2, where vol is volume, a the length of the major axis, and b the length of the minor axis.
Immunohistochemistry
Tumor samples excised from the animals were fixed for 1 hour in 10% neutral-buffered formalin at room temperature, washed overnight in PBS containing 10% sucrose at 4°C, and embedded in optimal cutting temperature compound (Tissue-Tek). The samples were then snap-frozen in dry-iced acetone for immunohistochemistry. Frozen samples were further sectioned at 10-μm thickness in a cryostat, briefly fixed with 10% formalin, and then incubated with primary and secondary antibodies. Samples were observed using an LSM510 Meta confocal microscope (Carl Zeiss). Quantification of CD31-stained areas was performed in multiple fields on tumor sections from 6 mice using Photoshop 8.0.1 software (Adobe Systems) and ImageJ 1.36b software (National Institutes of Health).
GEP analysis
Microarray data for NPM-ALK gene expression signature (GSE6184) and clinical gene expression profiling (GEP) data of peripheral T-cell lymphoma (PTCL) patient samples (GSE19069) were obtained from the National Center for Biotechnology Information's Gene Expression Omnibus.12,15 Gene-set enrichment analysis (GSEA) was performed with GSEA Version 2.0 software available from the Broad Institute (http://www.broadinstitute.org/gsea/) using microarray data for NPM-ALK signature (GSE6184) and GSEA-embedded potential miRNA target gene sets or TargetScan-predicted putative miRNA target lists (http://www.targetscan.org/).16
Statistical analysis
Statistical analysis was performed using the Student t test or multivariate ANOVA (for proliferation assay and in vivo analysis; *P < .05; **P < .01; ***P < .001). All data are expressed as mean ± SEM.
Results
Up-regulation of miR-135b in ALCL
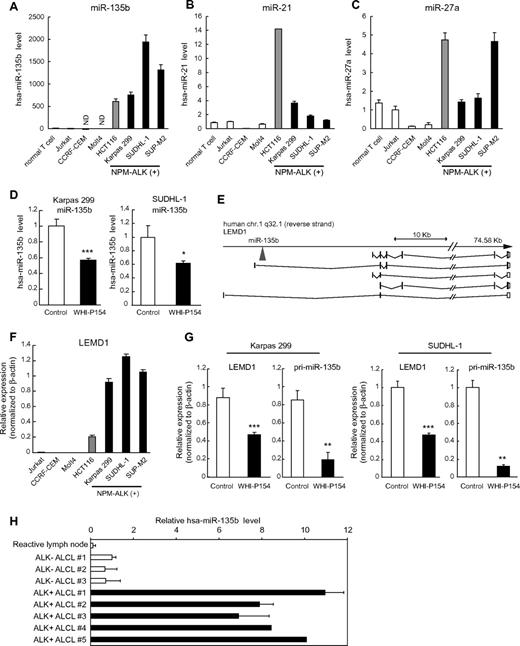
Previous microarray analysis on miRNA expression identified various signatures of aberrant miRNA expression in a wide range of hematologic cell lines, including major types of B- and T-cell lymphoma, lymphoproliferative disorder, T-cell acute lymphoblastic leukemia, and acute myeloid leukemia.17 In this analysis, ALCL was characterized by increased expression of miR-135b, miR-21, and miR-27a.17 We verified the observation using quantitative RT-PCR analysis on 3 ALCL cell lines carrying NPM-ALK fusion; Karpas 299, SUDHL-1, and SUP-M2 (Figure 1A-C). Among these miRNAs, miR-135b was most prominently up-regulated in ALCL cell lines, and it was reduced by the ALK inhibitor WHI-P154 (Figure 1D), prompting us to track potential downstream effector(s) of NPM-ALK to miR-135b. Because miR-135b is located in the first intron of the LEM domain containing 1 (LEMD1) gene on 1q32.1 (Figure 1E), we examined LEMD1 expression levels in ALCL cell lines. LEMD1 was also remarkably elevated in ALCL cells (Figure 1F). ALK inhibition resulted in a potent decrease of LEMD1 and primary transcript of miR-135b (pri-miR-135b) in Karpas 299 and SUDHL-1 cells (Figure 1G), as well as in mature miR-135b, suggesting that NPM-ALK characterizes high miR-135b expression in ALCL cells. The effects of WHI-P154 on mature miR-135b were lower than those on pri-miR-135b, possibly because of generally high stability of mature miRNAs. In addition, we measured miR135b expression levels in clinical samples of ALCL patients and found that miR-135b is elevated in human primary ALK-positive ALCL samples, compared with reactive lymph node and ALK-negative ALCL samples (Figure 1H).
High expression of miR-135b and LEMD1 in ALCL. (A-C) Expression of mature miR-135b (A), miR-21 (B), and miR-27a (C) in NPM-ALK (+) ALCL cell lines (Karpas 299, SUDHL-1, and SUP-M2), normal T lymphocytes, and several T-lymphoblastic leukemia cell lines (Jurkat, CCRF-CEM, and Molt4), detected by qRT-PCR analysis. HCT116 colon cancer cells expressing endogenous miR-135b were used as positive control. ND indicates not detected. (D) Attenuation of miR-135b expression by ALK inhibitor WHI-P154. Mature miR-135b in Karpas 299 and SUDHL-1 cells was analyzed by qRT-PCR after WHI-P154 treatment (10μM, 4 hours). (E) Schematic diagram of genomic organization of human LEMD1 gene and miR-135b. As for human LEMD1, several splicing variants have been reported. miR-135b is located in the first intron of LEMD1 longer transcripts. (F) High expression of LEMD1 in ALCL cells, determined as in panel A. (G) Suppression of LEMD1 and pri-miR-135b by WHI-P154 (10μM, 1 hour) in Karpas 299 and SUDHL-1 cells, assessed as in panel D (*P < .05; **P < .01; ***P < .001). (H) Expression of miR-135b in clinical samples of ALCL patients.
High expression of miR-135b and LEMD1 in ALCL. (A-C) Expression of mature miR-135b (A), miR-21 (B), and miR-27a (C) in NPM-ALK (+) ALCL cell lines (Karpas 299, SUDHL-1, and SUP-M2), normal T lymphocytes, and several T-lymphoblastic leukemia cell lines (Jurkat, CCRF-CEM, and Molt4), detected by qRT-PCR analysis. HCT116 colon cancer cells expressing endogenous miR-135b were used as positive control. ND indicates not detected. (D) Attenuation of miR-135b expression by ALK inhibitor WHI-P154. Mature miR-135b in Karpas 299 and SUDHL-1 cells was analyzed by qRT-PCR after WHI-P154 treatment (10μM, 4 hours). (E) Schematic diagram of genomic organization of human LEMD1 gene and miR-135b. As for human LEMD1, several splicing variants have been reported. miR-135b is located in the first intron of LEMD1 longer transcripts. (F) High expression of LEMD1 in ALCL cells, determined as in panel A. (G) Suppression of LEMD1 and pri-miR-135b by WHI-P154 (10μM, 1 hour) in Karpas 299 and SUDHL-1 cells, assessed as in panel D (*P < .05; **P < .01; ***P < .001). (H) Expression of miR-135b in clinical samples of ALCL patients.
NPM-ALK induces LEMD1/miR-135b through STAT3 activation
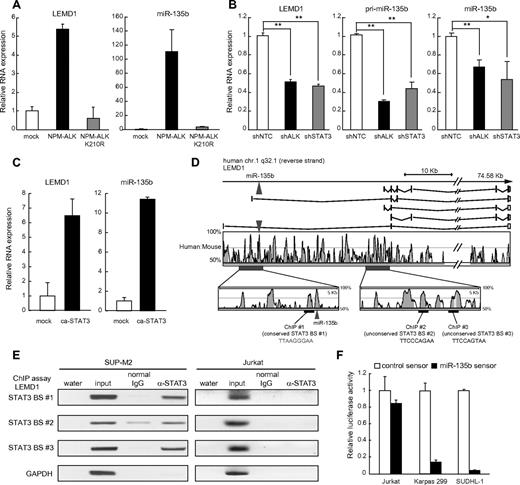
To examine a direct involvement of NPM-ALK in miR-135b up-regulation in ALCL cells, we introduced wild-type and kinase-dead NPM-ALK into the human Jurkat cells and examined the expression levels of LEMD1 and miR-135b. NPM-ALK but not kinase-dead NPM-ALK (K210R) induced both LEMD1 and mature miR-135b in Jurkat cells (Figure 2A), indicating that NPM-ALK up-regulates the host gene and subsequently miR-135b through the kinase activity.
Identification of NPM-ALK-STAT3-miR-135b axis in ALCL. (A) Induction of LEMD1/miR-135b by NPM-ALK. Jurkat cells were transduced with lentivirus carrying NPM-ALK or kinase-dead NPM-ALK (K210R) and subjected to qRT-PCR analysis. (B) NPM-ALK/STAT3–dependent up-regulation of LEMD1/miR-135b in ALCL. Effects of shRNAs against NPM-ALK and STAT3 were evaluated in SUDHL-1 cells (*P < .05; **P < .01). (C) Up-regulation of LEMD1/miR-135b by ca-STAT3. Jurkat cells were transduced with lentivirus carrying mouse ca-STAT3 and subjected to qRT-PCR analysis. (D) STAT3 binding sites in LEMD1 genomic region. Top panel indicates schematic genomic organization of human LEMD1 gene and miR-135b. Sequence conservation between human and mouse is represented as the percentage of conservation in the Vista analysis shown in the middle panel. We analyzed putative STAT3-binding sites within the conserved region in ChIP analysis, and we found that STAT3 bound to 3 sites in the bottom panel, as shown in panel E. Number 1 site (TTAAGGGAA) is conserved between human and mouse. (E) Binding of STAT3 to LEMD1 genomic regions analyzed by ChIP analysis in SUP-M2 (left) and Jurkat (right) cells. Total chromatin before immunoprecipitation was used as positive control for PCR. Jurkat cells were used as negative control. (F) Enhanced miR-135b activity in ALCL. Cells were transfected with miRNA sensor vectors and applied to luciferase assay. Reflecting high miR-135b expression, luciferase expression levels from miR-135b sensor vector were remarkably lower than those from control sensor vector in ALCL cells but not in Jurkat cells.
Identification of NPM-ALK-STAT3-miR-135b axis in ALCL. (A) Induction of LEMD1/miR-135b by NPM-ALK. Jurkat cells were transduced with lentivirus carrying NPM-ALK or kinase-dead NPM-ALK (K210R) and subjected to qRT-PCR analysis. (B) NPM-ALK/STAT3–dependent up-regulation of LEMD1/miR-135b in ALCL. Effects of shRNAs against NPM-ALK and STAT3 were evaluated in SUDHL-1 cells (*P < .05; **P < .01). (C) Up-regulation of LEMD1/miR-135b by ca-STAT3. Jurkat cells were transduced with lentivirus carrying mouse ca-STAT3 and subjected to qRT-PCR analysis. (D) STAT3 binding sites in LEMD1 genomic region. Top panel indicates schematic genomic organization of human LEMD1 gene and miR-135b. Sequence conservation between human and mouse is represented as the percentage of conservation in the Vista analysis shown in the middle panel. We analyzed putative STAT3-binding sites within the conserved region in ChIP analysis, and we found that STAT3 bound to 3 sites in the bottom panel, as shown in panel E. Number 1 site (TTAAGGGAA) is conserved between human and mouse. (E) Binding of STAT3 to LEMD1 genomic regions analyzed by ChIP analysis in SUP-M2 (left) and Jurkat (right) cells. Total chromatin before immunoprecipitation was used as positive control for PCR. Jurkat cells were used as negative control. (F) Enhanced miR-135b activity in ALCL. Cells were transfected with miRNA sensor vectors and applied to luciferase assay. Reflecting high miR-135b expression, luciferase expression levels from miR-135b sensor vector were remarkably lower than those from control sensor vector in ALCL cells but not in Jurkat cells.
Because previous reports demonstrated that NPM-ALK elicits many downstream pathways, including STAT3, Ras-ERK, and PI3K signaling pathways,9 we next examined the involvement of these pathways. Knockdown of NPM-ALK or STAT3 suppressed the expression of LEMD1 and miR-135b in ALCL cells (Figure 2B and supplemental Figure 1A),12,13 whereas inhibition of ERK or PI3K failed to exert such effects (supplemental Figure 1B). We also confirmed that ca-STAT3 up-regulates LEMD1 and miR-135b in Jurkat cells (Figure 2C). Furthermore, we analyzed putative STAT3-binding sites within the conserved region of LEMD1 gene between human and mouse and showed that STAT3 binds to putative STAT3-binding sites within the LEMD1 genomic region by chromatin-immunoprecipitation analysis (Figure 2D-E). Reflecting high miR-135b expression, luciferase expression levels from miR-135b sensor vector were remarkably lower than those from control sensor vector in ALCL cells but not in Jurkat cells, confirming that miR-135b is highly active in ALCL cells (Figure 2F). Taken together, these results demonstrate that miR-135b lies downstream of the NPM-ALK/STAT3 signaling pathway in ALCL.
miR-135b targets FOXO1 and regulates chemosensitivity
Several potential targets of miR-135b with tumor-suppressive activities were computationally predicted previously18 and included APC, LZTS1, LATS2, CREG1, and FOXO1. APC has been already shown as a target of miR-135b,19 and we validated LZTS1, LATS2, and FOXO1 using luciferase reporter assay (supplemental Figure 2A-B). To identify the endogenous and functional target(s) of miR-135b in ALCL cells, we achieved the efficient long-term suppression of miR-135b activity through the decoy RNA system, in which RNA decoys against specific miRNA (TuD RNA) are driven by RNA polymerase III (Figure 3A).11 Introduction of TuD RNA indeed strongly inhibited miR-135b activity in ALCL cell lines (Figure 3B-C and supplemental Figure 2C-D).
miR-135b targets FOXO1 and regulates chemosensitivity. (A) Structure of TuD RNA expression vector. TuD RNA for miR-135b contains miRNA-binding site (MBS) that is partially complementary to miR-135b. Details of TuD RNA structure have been described previously.11 (B) Introduction of TuD RNA against miR-135b into ALCL cells. Flow cytometry profiles indicating transduction efficiency in Karpas 299 cells. FSC indicates forward scatter. (C) Inhibition of miR-135b function by TuD RNA for miR-135b. Left and right panels show qRT-PCR result and luciferase assay monitoring miR-135b activity, respectively. NC indicates negative control. (D) Sequence alignment between miR-135b and its putative binding site in the FOXO1 3′UTR. (E) miR-135b targets FOXO1. HEK293T cells were transfected with luciferase reporter containing the FOXO1 3′UTR with wild-type or mutated target site (shown in panel D), along with empty vector, miRNA-lacZ expression control vector (miRNA for lacZ), or pri-miR-135b expression vector [miR-135b (+)]. Luciferase assay was performed 48 hours after transfection (***P < .001). (F) Suppression of FOXO1 protein level by miR-135b. HeLa cells were transiently transfected with miR-135b and subjected to immunoblot analysis. (G) Elevated expression of FOXO1, p21, and p27 by miR-135b inhibition in ALCL cells. SUDHL-1 and SUP-M2 cells were infected with lentivirus harboring TuD-NC or TuD-miR-135b and applied to immunoblot analysis. The quantification results were shown in the bottom panel. (H) miR-135b–mediated attenuation of chemosensitivity. Jurkat cells with empty or pri-miR-135b vectors were treated with cytosine β-d-arabinofuranoside (AraC), followed by the assessment of cell viability (left) and apoptosis (right; *P < .05; ***P < .001; n.s., not significant).
miR-135b targets FOXO1 and regulates chemosensitivity. (A) Structure of TuD RNA expression vector. TuD RNA for miR-135b contains miRNA-binding site (MBS) that is partially complementary to miR-135b. Details of TuD RNA structure have been described previously.11 (B) Introduction of TuD RNA against miR-135b into ALCL cells. Flow cytometry profiles indicating transduction efficiency in Karpas 299 cells. FSC indicates forward scatter. (C) Inhibition of miR-135b function by TuD RNA for miR-135b. Left and right panels show qRT-PCR result and luciferase assay monitoring miR-135b activity, respectively. NC indicates negative control. (D) Sequence alignment between miR-135b and its putative binding site in the FOXO1 3′UTR. (E) miR-135b targets FOXO1. HEK293T cells were transfected with luciferase reporter containing the FOXO1 3′UTR with wild-type or mutated target site (shown in panel D), along with empty vector, miRNA-lacZ expression control vector (miRNA for lacZ), or pri-miR-135b expression vector [miR-135b (+)]. Luciferase assay was performed 48 hours after transfection (***P < .001). (F) Suppression of FOXO1 protein level by miR-135b. HeLa cells were transiently transfected with miR-135b and subjected to immunoblot analysis. (G) Elevated expression of FOXO1, p21, and p27 by miR-135b inhibition in ALCL cells. SUDHL-1 and SUP-M2 cells were infected with lentivirus harboring TuD-NC or TuD-miR-135b and applied to immunoblot analysis. The quantification results were shown in the bottom panel. (H) miR-135b–mediated attenuation of chemosensitivity. Jurkat cells with empty or pri-miR-135b vectors were treated with cytosine β-d-arabinofuranoside (AraC), followed by the assessment of cell viability (left) and apoptosis (right; *P < .05; ***P < .001; n.s., not significant).
Using this system, we consequently identified FOXO1 as an endogenous target of miR-135b in ALCL (Figure 3D-G). The FOXO1 3′UTR contains a potential miR-135b–binding site and exogenous miR-135b suppressed FOXO1 protein expression and its translational efficiency depending on the target site, clarifying FOXO1 as a novel target of miR-135b (Figure 3D-F). Knockdown of miR-135b increased the protein expression of FOXO-dependent cell cycle inhibitors p21 and p27 as well as FOXO1 itself in ALCL cells (Figure 3G and supplemental Figure 2E). In Karpas 299 cells containing a barely detectable FOXO1, miR-135b suppression up-regulated p27 and alternatively another positive regulator of p27, CREG1 (supplemental Figure 2E).20 Considering that NPM-ALK has been shown to inhibit FOXO3a activity through FOXO3a phosphorylation by AKT activation,21 NPM-ALK might thus regulate a wide range of FOXO family activities via protein modification and posttranscriptional regulation. Because FOXO factors are critical mediators in growth inhibitory responses to various stresses, including DNA damage,22 we examined the effect of miR-135b on the sensitivity to chemotherapeutic drugs to investigate functional consequences of FOXO1 alteration by miR-135b. Although miR-135 overexpression did not affect the proliferation of Jurkat cells under normal conditions, Jurkat cells overexpressing miR-135b were more resistant to cytosine β-d-arabinofuranoside (Figure 3H). These results suggest a possibility that miR-135b may confer chemoresistance to ALCL cells through FOXO1 modulation.
Targeting of Th2 regulators STAT6 and GATA3 by miR-135b
It was shown previously that constitutive activation of ALK chimeric proteins is sufficient to induce cellular transformation and that ALK activity is indispensable for the survival of ALK-positive ALCL cells.9 In the pathogenesis of ALCL, ALK elicits reproducible transcriptome changes, as shown by previous GEP analysis.12 To gain insight for overall interconnection between NPM-ALK–driven gene response and miR-135b–mediated gene regulation, we performed GSEA using the GEP results of ALCL cells with or without shRNA-mediated NPM-ALK inhibition.12,16 GSEA demonstrated a significant up-regulation of miR-135b potential targets on NPM-ALK suppression, indicating that miR-135b constitutes an arm of multiple NPM-ALK downstream pathways (Figure 4A and supplemental Figure 3A). This approach suggested TGFBR1, SIRT1, cyclin G2, CREG1, Bcl11b, and STAT6 as additional candidate targets of the NPM-ALK–miR-135b pathway.
Targeting of STAT6 and GATA3 by miR-135b in ALCL. (A) Up-regulation of miR-135b target genes was evaluated on NPM-ALK knockdown using GSEA (dataset GSE6184). (B) Heat map showing the expression of Th17-related molecules, GATA3, FOXO1, and other ALCL-related genes (GZMB, PRF1, and TCR-related genes) in PTCL. Gene expression data are derived from GSE19069. In addition to GATA3, FOXO1 expression in ALCL was also lower than those in other PTCLs. ATLL indicates adult T-cell leukemia/lymphoma; AITL, angioimmunoblastic T-cell lymphoma; and PTCLu, PTCL-unclassifiable. (C) Sequence alignment between miR-135b and its putative binding sites in the STAT6 and GATA3 3′UTRs. (D) miR-135b targets STAT6 and GATA3. Luciferase activity of the STAT6 and GATA3 3′UTR reporter constructs with wild-type or mutated target site (shown in panel C) in HEK293T cells cotransfected with empty vector, miRNA-lacZ expression control vector (miRNA for lacZ), or pri-miR-135b expression vector [miR-135b (+)]; ***P < .001). (E) Up-regulation of STAT6 and GATA3 by TuD-RNA–mediated miR-135b inhibition in Karpas 299 cells. The quantification results were shown in the right panel.
Targeting of STAT6 and GATA3 by miR-135b in ALCL. (A) Up-regulation of miR-135b target genes was evaluated on NPM-ALK knockdown using GSEA (dataset GSE6184). (B) Heat map showing the expression of Th17-related molecules, GATA3, FOXO1, and other ALCL-related genes (GZMB, PRF1, and TCR-related genes) in PTCL. Gene expression data are derived from GSE19069. In addition to GATA3, FOXO1 expression in ALCL was also lower than those in other PTCLs. ATLL indicates adult T-cell leukemia/lymphoma; AITL, angioimmunoblastic T-cell lymphoma; and PTCLu, PTCL-unclassifiable. (C) Sequence alignment between miR-135b and its putative binding sites in the STAT6 and GATA3 3′UTRs. (D) miR-135b targets STAT6 and GATA3. Luciferase activity of the STAT6 and GATA3 3′UTR reporter constructs with wild-type or mutated target site (shown in panel C) in HEK293T cells cotransfected with empty vector, miRNA-lacZ expression control vector (miRNA for lacZ), or pri-miR-135b expression vector [miR-135b (+)]; ***P < .001). (E) Up-regulation of STAT6 and GATA3 by TuD-RNA–mediated miR-135b inhibition in Karpas 299 cells. The quantification results were shown in the right panel.
ALCL of T-lymphocyte origin has been known to present with a T- or null-cell phenotype and lack TCR complex-related molecules such as CD3ϵ and ZAP70, despite the presence of TCR rearrangements (Figure 4B).23 Alternatively, recent GEP analysis in PTCL has demonstrated the following characters of ALCL cells: high expression of Th17-cell–associated molecules (IL-17A, IL-17F, and retinoic acid-related orphan receptor [ROR]γ), overlapping with Th17 cells phenotype, low expression of GATA3, and suppression of TCR components, as summarized in Figure 4B.15 Previous studies also have demonstrated that both Th1 and Th2 differentiation programs antagonize Th17-cell differentiation.24
Along with these observations, reassessment of computational prediction proposed that miR-135b potentially targets 2 Th2 master regulators GATA3 and STAT6 (Figure 4C and supplemental Figure 3B). The GSEA result also supported the possibility of STAT6 as a miR-135b target (supplemental Figure 3A). We thus investigated the immune modulatory property of miR-135b on ALCL immunophenotype overlapping with Th17 cells. Luciferase assays revealed that miR-135b targets the 3′UTRs of both STAT6 and GATA3 (Figure 4D). Mutagenesis of potential target sites in their 3′UTR abrogated the response of STAT6 and GATA3 3′UTR to miR-135b, confirming direct interactions of miR-135b with STAT6 and GATA3 (Figure 4D). We further found that suppression of miR-135b indeed up-regulates protein expression of STAT6 and GATA3 in ALCL cells, demonstrating that both STAT6 and GATA3 are intrinsic targets of miR-135b in ALCL cells (Figure 4E and supplemental Figure 3C).
miR-135b blockade suppresses IL-17 production by ALCL cells
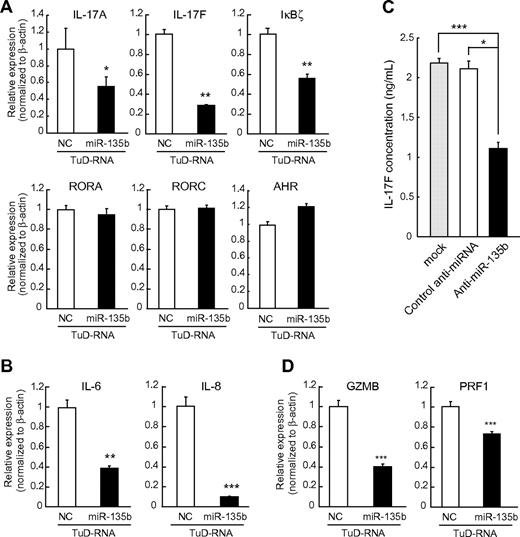
IL-4–STAT6 axis and GATA3 are important for Th2 differentiation of normal lymphocytes.25 In normal lymphocyte differentiation, lineage-specific transcription factors can both activate and repress differentiation programs.26,27 GATA3 simultaneously promotes Th2 differentiation and represses Th1 differentiation, because Th1 regulator T-bet has opposing bidirectional effects.25 A similar relationship also exists between Th1 and Th2 differentiaton programs and Th17 differentiation programs. As Th1 and Th2 effector cytokines (IFN-γ and IL-4) antagonize Th17 differentiation,24 both GATA3 and T-bet have been shown to suppress Th17 differentiation.27,28 On the basis of these concepts, we next examined the effects of miR-135b suppression on the expressions of Th17-related molecules. Karpas 299 ALCL cells endogenously expressed IL-17, and miR-135b suppression attenuated the expression levels of IL-17A and IL-17F transcripts (Figure 5A), consistent with GATA3-mediated suppression of Th17 differentiation.28 Blockade of miR-135b also suppressed the expression of IκBζ, a recently identified key regulator of Th17 differentiation,29 without concomitant changes of RORγ, RORα, or aryl hydrocarbon receptor. In addition, down-regulation of proinflammatory cytokines including IL-6 and IL-8 was observed by miR-135b suppression (Figure 5B). Consistently, we confirmed that miR-135b suppression attenuates IL-17 production in Karpas 299 cells (Figure 5C). Taken together, these findings suggest that NPM-ALK/STAT3–miR-135b axis polarizes the identity of ALCL cells to the IL-17–producing immunophenotype resembling Th17 cells by suppression of GATA3 and STAT6. In addition, miR-135b knockdown suppressed the expression of granzyme B and perforin 1, cytotoxic molecules highly expressed in ALCL (Figure 5D),15 suggesting that miR-135b affects the broad range of ALCL immunophenotype.
miR-135b blockade suppresses IL-17 production by ALCL cells and modulates ALCL immunophenotype. (A) Regulation of Th17-related molecules by miR-135b. Karpas 299 cells were infected with lentivirus harboring TuD-NC or TuD-miR-135b and applied to qRT-PCR analysis. Effects of miR-135b inhibition on the transcripts of Th17-related molecules were analyzed by qRT-PCR assay. Effects of miR-135b inhibition on IL-6 and IL-8 expression, as determined in panel A. (C) Effects of miR-135b inhibition on IL-17F production were analyzed in Karpas 299 cells by ELISA. (D) Effects of miR-135b inhibition on granzyme B (GZMB) and perforin 1 (PRF1) expression, as determined in panel A. miR-135b blockade did not affect the expression levels of IL-21, IL-23, and TGF-β1 (data not shown; *P < .05; **P < .01; ***P < .001).
miR-135b blockade suppresses IL-17 production by ALCL cells and modulates ALCL immunophenotype. (A) Regulation of Th17-related molecules by miR-135b. Karpas 299 cells were infected with lentivirus harboring TuD-NC or TuD-miR-135b and applied to qRT-PCR analysis. Effects of miR-135b inhibition on the transcripts of Th17-related molecules were analyzed by qRT-PCR assay. Effects of miR-135b inhibition on IL-6 and IL-8 expression, as determined in panel A. (C) Effects of miR-135b inhibition on IL-17F production were analyzed in Karpas 299 cells by ELISA. (D) Effects of miR-135b inhibition on granzyme B (GZMB) and perforin 1 (PRF1) expression, as determined in panel A. miR-135b blockade did not affect the expression levels of IL-21, IL-23, and TGF-β1 (data not shown; *P < .05; **P < .01; ***P < .001).
Modulation of paracrine inflammatory reaction and tumorigenic potential of ALCL by miR-135b
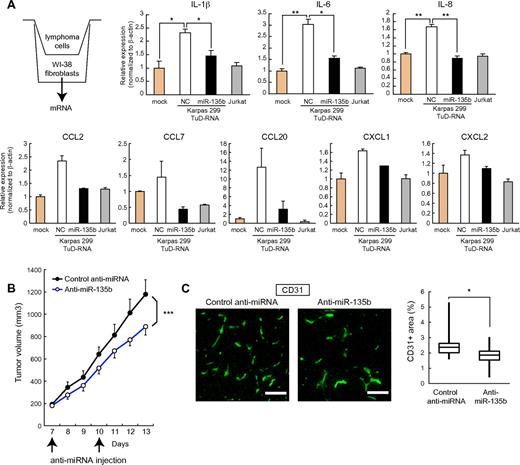
IL-17 is a proinflammatory cytokine that stimulates the production of various inflammatory cytokines (eg, IL-1β, IL-6, IL-8, G-CSF, and GM-CSF) and chemokines (eg, CCL2, CCL7, CCL20, CXCL1, and CXCL2) from many cell types, such as fibroblasts, endothelial cells, and neutrophils, and is involved in pathogenesis of autoimmune disorders.30 Although the significance of IL-17 in cancer is largely unknown and might depend on cancer-type and context, proinflammatory and proangiogenic properties of IL-17 have been associated with tumor progression in several studies.31-34 In this setting, IL-17 has been shown to augment the secretion of angiogenic chemokines, such as CXCL1 and CXCL5.34 We analyzed the operational role of miR-135b in tumor development of ALCL. Accordingly, coculture experiments demonstrated that Karpas 299 cells stimulate the production of proinflammatory cytokines (IL-1β, IL-6, and IL-8) and chemokines (CCL2, CCL7, CCL20, and CXCL1/2) in WI-38 human fibroblasts in an miR-135b–dependent manner (Figure 6A), underscoring the involvement of miR-135b in paracrine inflammatory response.
Regulation of paracrine inflammatory reaction and tumorigenic potential by miR-135b. (A) ALCL cells stimulate proinflammatory cytokine (IL-1β, IL-6, and IL-8) and chemokine (CCL2, CCL7, CCL20, CXCL1, and CXCL2) production in human fibroblasts through an miR-135b–dependent manner. WI-38 human fibroblasts were cocultured with Karpas 299 or Jurkat cells and subjected to qRT-PCR assay (*P < .05; **P < .01). (B) Growth curves of Karpas 299 subcutaneous tumors after transplantation into SCID mice and administration with miR-135 antisense–atelocollagen or control oligonucleotide–atelocollagen complexes. After random assignment at day 7 after inoculation (n = 6/group), LNA-based antisense oligonucleotides (5μM) with atelocollagen were administered into the subcutaneous spaces around the tumors at days 7 and 10 and measured (means ± SEM; ***P < .001). (C) Effects of miR-135b inhibition on tumor angiogenesis. Representative images of CD31 immunostaining of tumor sections (left) and quantification of microvessel density measured by CD31-positive area (right). Pixel density was quantified in multiple tumor images from 6 mice per group using ImageJ 1.36b software. Scale bar represents 100 μm (*P < .05).
Regulation of paracrine inflammatory reaction and tumorigenic potential by miR-135b. (A) ALCL cells stimulate proinflammatory cytokine (IL-1β, IL-6, and IL-8) and chemokine (CCL2, CCL7, CCL20, CXCL1, and CXCL2) production in human fibroblasts through an miR-135b–dependent manner. WI-38 human fibroblasts were cocultured with Karpas 299 or Jurkat cells and subjected to qRT-PCR assay (*P < .05; **P < .01). (B) Growth curves of Karpas 299 subcutaneous tumors after transplantation into SCID mice and administration with miR-135 antisense–atelocollagen or control oligonucleotide–atelocollagen complexes. After random assignment at day 7 after inoculation (n = 6/group), LNA-based antisense oligonucleotides (5μM) with atelocollagen were administered into the subcutaneous spaces around the tumors at days 7 and 10 and measured (means ± SEM; ***P < .001). (C) Effects of miR-135b inhibition on tumor angiogenesis. Representative images of CD31 immunostaining of tumor sections (left) and quantification of microvessel density measured by CD31-positive area (right). Pixel density was quantified in multiple tumor images from 6 mice per group using ImageJ 1.36b software. Scale bar represents 100 μm (*P < .05).
We also examined the roles of miR-135b in ALCL tumorigenic potential in vivo. In a xenograft model, local administration of LNA-based miRNA inhibitors against miR-135b with atelocollagen suppressed the growth of subcutaneous Karpas 299 tumors (Figure 6B). Furthermore, the inhibition of in vivo tumor growth by anti–miR-135b antisense was accompanied with reduced tumor angiogenesis (Figure 6C). These results thus collectively suggest that miR-135b–mediated modulation of paracrine inflammatory reaction favors tumor microenvironment, also indicating a therapeutic significance of targeting this axis by miR-135b interference.
Discussion
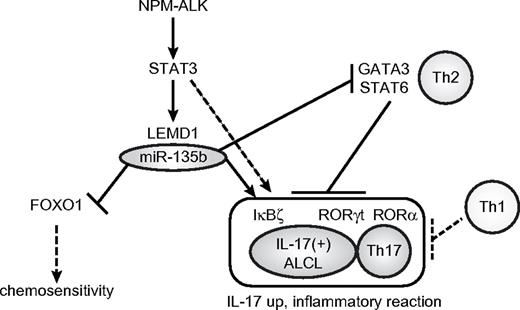
Here, we identified miR-135b as a downstream mediator of NPM-ALK/STAT3 signaling (Figure 7). Although NPM-ALK drives malignant transformation of ALCL cells through various downstream signaling pathways, including STAT3, Ras-ERK, and PI3K,9 our study demonstrated the oncogenic aspect of miR-135b targeting FOXO1 in ALCL pathogenesis (Figure 7). It was shown previously that miR-135b is highly expressed in embryonic stem cells and other cancer types, including colorectal and prostate cancer.19,35,36 Considering the essential roles in embryonic stem cells and the pivotal oncogenic functions of STAT3,37 the STAT3–miR-135b pathway might be widely used in these conditions. Oncogenic roles of miR-135b also might be supported by up-regulation of its host LEMD1, which is alternatively known as cancer/testis antigen 50, and DNA copy number gain in 1q32.1 in colorectal tumors.38-40 Although the biologic function(s) of LEMD1 has not been investigated so far, LEMD1 also might contribute to NPM-ALK–driven oncogenicity and IL-17–producing immunophenotype in ALCL. Considering other reports linking NPM-ALK to inhibition of FOXO3a activity through PI3K/Akt pathway,21 NPM-ALK might engage multiple players, including noncoding RNAs to prevent these central tumor suppressor pathways from activating by NPM-ALK–induced oncogenic stresses. We also examined miR-135b expression levels in lung cancer and neuroblastoma cell lines carrying ALK abnormalities (supplemental Table 4 and Figure 4). miR-135b expression was very low in neuroblastoma cell lines examined. The H2228 lung adenocarcinoma cell line containing EML4-ALK fusion expressed miR-135b to some extent, although at a lower level than ALCL cells. Involvement of miR-135b in the pathogenesis of some other tumor subtypes engaging ALK abnormalities remains to be further elucidated.
Summary of roles of miR-135b in ALCL pathogenesis. The present study demonstrates that NPM-ALK induces LEMD1 and miR-135b expression through STAT3 in ALCL and that miR-135b targets FOXO1 and two versatile Th2 regulators, GATA3, and STAT6, rendering the IL-17–producing immunophenotype to ALCL.
Summary of roles of miR-135b in ALCL pathogenesis. The present study demonstrates that NPM-ALK induces LEMD1 and miR-135b expression through STAT3 in ALCL and that miR-135b targets FOXO1 and two versatile Th2 regulators, GATA3, and STAT6, rendering the IL-17–producing immunophenotype to ALCL.
Importantly, we uncovered an interesting immune modulatory property of miR-135b. miR-135b targeted Th2 master regulators STAT6 and GATA3, and inhibition of miR-135b suppressed IL-17 production by ALCL cells, evidencing the Th17-skewing effect of miR-135b. ALK-positive ALCL is characterized by the presence of NPM-ALK and was originally described as a T- or null-cell phenotype. Loss of T-cell phenotype frequently observed in ALCL, shown by decreased expression of αβ-TCR heterodimer and CD3ϵ, has been demonstrated to be partly mediated by the perturbation by NPM-ALK signaling.23 Conversely, it is postulated that the normal counterpart of ALCL is an activated mature cytotoxic T-cell, because of the expression of cytotoxic granule-associated molecules such as TIA1, gramzyme B, and perforin 1, although CD8 is usually negative in ALCL and CD4 is positive in 70% cases of ALCL.41 In addition to this aberrant phenotype, a recent molecular signature study of PTCL revealed that ALCL displays up-regulation of Th17-cell–associated molecules (IL-17A, IL-17F, and RORγ) and down-regulation of GATA3. The immunophenotype overlaps with that of Th17 cells, compared with other lymphoma subtypes such as angioimmunoblastic T-cell lymphoma (AITL), ALK-negative ALCL, adult T-cell leukemia/lymphoma, and PTCL-unclassifiable.15 In support of this notion, a most recent report demonstrates that levels of IL-17, IL-8, and IL-22 were elevated in untreated ALK-positive ALCL patients' sera but undetectable in those of complete remission after chemotherapy,42 reinforcing the relevance of IL-17–producing immunophenotype of ALCL. Although the underlying mechanisms determining the immunophenotypes of various lymphomas have been largely unresolved, our study has demonstrated that a signaling network elicited by ectopic expression of NPM-ALK oncogene and its downstream miRNA confers the aberrant immunophenotype of ALCL cells.
Importance of tumor microenvironment in lymphoma development has been well investigated in B-cell lymphoma, including Hodgkin lymphoma.43 A recent report has shown that IL-17–positive cells were more frequently observed in AITL than in PTCL-unclassifiable and that both Th17 and mast cells contribute to the lymphoma-associated proinflammatory environment in AITL.44 In contrast, ALCL is unique in the respect that ALCL cells produce IL-17 by themselves, which might result in different clinical features between these lymphoma subtypes. In consistent with strong effects of IL-17 on neutrophils, extensive neutrophil infiltration has been observed in some cases of ALCL, as referred to as neutrophil-rich ALCL, which can sometimes be misdiagnosed as an inflammatory disease rather than lymphoma.45-47 Frequent B symptoms in ALCL also might be attributable to this IL-17–producing immunophenotype. In this study, antisense-based miR-135b blockade reduced tumor angiogenesis and growth in an in vivo tumor model, which observation is consistent with the proangiogenic function of IL-17 (Figure 6).33,34 Although a line of observations suggest both pro- and antitumor potentials of IL-17 or Th17 cells in a context-dependent manner,31,32 the tumor-suppressive effect of miR-135b inhibition suggests that local proinflammatory properties of IL-17–producing ALCL cells may provide the favorable tumor microenvironment through the enhancement of angiogenesis or so in this context. Recent evidence has revealed that many characteristic aspects of cancer-related biologic processes, including drug resistance, maintenance of cancer-initiating cells, and metastasis, are associated with miRNA function.48 The present study demonstrates another unique contribution of miRNA dysregulation to tumor development, ie, modulation of immunophenotype of lymphoma cells and the consequent alteration of tumor milieu by miR-135b.
Among hundreds of miRNAs, miR-326 has been identified so far as a regulator of Th17 differentiation, which promoted Th17 differentiation via targeting ETS1.49 In addition, miR-155 was recently shown to promote Th17 cell formation in a CD4+ T-cell intrinsic manner.50 It was consistent with the finding that miR-155 targets Th2 promoter c-Maf. Our study suggests the presence of additional regulatory miRNAs of Th17 differentiation through the inhibition of differentiation program(s) to other helper T-cell lineage(s). Generally, lineage-specific transcription factors and cytokines can interfere with the differentiation to other helper T-cell subsets. Although it requires careful assessment about difference between normal lymphocyte differentiation and lymphoma cell phenotype polarization, our findings suggest that miRNAs might be widely involved in this reciprocal regulatory network between helper T-cell differentiation programs. The STAT3–miR-135b–GATA3/STAT6 connection revealed in this study might propose inhibitory mechanism(s) against Th2 in Th17 differentiation program, mirroring the opposite inhibitory impacts of Th1 and Th2 programs on Th17 differentiation,24,26 for ensuring mutual exclusion.
In conclusion, our study demonstrates a novel oncogenic pathway composed of NPM-ALK, STAT3, and miR-135b that authorizes IL-17–producing immunophenotype of ALCL. Tumor suppression with miR-135b blockade also demonstrates the therapeutic potential of miR-135b interference strategy targeting the “Th17 mimic” axis. These findings might advance our understandings of ALK-mediated oncogenesis and be useful for the development of new therapeutic interventions.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank E. Johansson, Y. Morishita, K. Kiyono, M. Morikawa, Y. Yoshimatsu, K. Isogaya, and H. Mihira for discussion and skilled technical assistance and all members of the Department of Molecular Pathology, University of Tokyo.
This work was supported by KAKENHI (Grants-in-Aid for Scientific Research for Research Activity start-up 22890038 and Innovative Areas “RNA regulation” 23112702); the Global Center of Excellence Program for “Integrative Life Science Based on the Study of Biosignaling Mechanisms” from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and the Cell Science Research Foundation.
Authorship
Contribution: H. Matsuyama and H.I.S. conceived and designed the research; H. Matsuyama performed experiments and analyses and wrote the paper; H.I.S. provided key materials and analyzed and wrote the paper; H.N. performed animal experiments and analysis; M.N., T.Y., N.K., H. Mano and K.S. provided clinical samples; and K.S. and K.M. supervised the whole project and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kohei Miyazono, Department of Molecular Pathology, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; e-mail: miyazono@m.u-tokyo.ac.jp.
References
Author notes
H.M. and H.I.S. contributed equally to this study.

![Figure 3. miR-135b targets FOXO1 and regulates chemosensitivity. (A) Structure of TuD RNA expression vector. TuD RNA for miR-135b contains miRNA-binding site (MBS) that is partially complementary to miR-135b. Details of TuD RNA structure have been described previously.11 (B) Introduction of TuD RNA against miR-135b into ALCL cells. Flow cytometry profiles indicating transduction efficiency in Karpas 299 cells. FSC indicates forward scatter. (C) Inhibition of miR-135b function by TuD RNA for miR-135b. Left and right panels show qRT-PCR result and luciferase assay monitoring miR-135b activity, respectively. NC indicates negative control. (D) Sequence alignment between miR-135b and its putative binding site in the FOXO1 3′UTR. (E) miR-135b targets FOXO1. HEK293T cells were transfected with luciferase reporter containing the FOXO1 3′UTR with wild-type or mutated target site (shown in panel D), along with empty vector, miRNA-lacZ expression control vector (miRNA for lacZ), or pri-miR-135b expression vector [miR-135b (+)]. Luciferase assay was performed 48 hours after transfection (***P < .001). (F) Suppression of FOXO1 protein level by miR-135b. HeLa cells were transiently transfected with miR-135b and subjected to immunoblot analysis. (G) Elevated expression of FOXO1, p21, and p27 by miR-135b inhibition in ALCL cells. SUDHL-1 and SUP-M2 cells were infected with lentivirus harboring TuD-NC or TuD-miR-135b and applied to immunoblot analysis. The quantification results were shown in the bottom panel. (H) miR-135b–mediated attenuation of chemosensitivity. Jurkat cells with empty or pri-miR-135b vectors were treated with cytosine β-d-arabinofuranoside (AraC), followed by the assessment of cell viability (left) and apoptosis (right; *P < .05; ***P < .001; n.s., not significant).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/26/10.1182_blood-2011-05-354654/4/m_zh89991183460003.jpeg?Expires=1769113760&Signature=UJZgpxjgWZGkWn6sour937enlj04pCWbRB67DY1pFaYmHi1RlgYkl3hQjrzncewpMGAPd5mrfLGpi9Z32aLL9XHtztt0OOH76PTAPLkWFDM946D~WY3O5GyIwNq-NUcX9qzLj4Ky8zjON6nydgjYKxnkA-OpPHqcYuBTn9xvKOmBEINGfv5I1Ck3uQk~NbQQGswwXJIkRBQtnud-9jVR~q8fi90-7iNOtwrf-eXIyvF0KgF1gnqvjUi57TUc0ZmO4o6~8pax8~3vFE9QhDSpfO~rbSkhRr9LIPm7CmVSH8DyLAhZYMa4jplFN~1Un9CE1tjO47U0UKor3fjJZzhDiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Targeting of STAT6 and GATA3 by miR-135b in ALCL. (A) Up-regulation of miR-135b target genes was evaluated on NPM-ALK knockdown using GSEA (dataset GSE6184). (B) Heat map showing the expression of Th17-related molecules, GATA3, FOXO1, and other ALCL-related genes (GZMB, PRF1, and TCR-related genes) in PTCL. Gene expression data are derived from GSE19069. In addition to GATA3, FOXO1 expression in ALCL was also lower than those in other PTCLs. ATLL indicates adult T-cell leukemia/lymphoma; AITL, angioimmunoblastic T-cell lymphoma; and PTCLu, PTCL-unclassifiable. (C) Sequence alignment between miR-135b and its putative binding sites in the STAT6 and GATA3 3′UTRs. (D) miR-135b targets STAT6 and GATA3. Luciferase activity of the STAT6 and GATA3 3′UTR reporter constructs with wild-type or mutated target site (shown in panel C) in HEK293T cells cotransfected with empty vector, miRNA-lacZ expression control vector (miRNA for lacZ), or pri-miR-135b expression vector [miR-135b (+)]; ***P < .001). (E) Up-regulation of STAT6 and GATA3 by TuD-RNA–mediated miR-135b inhibition in Karpas 299 cells. The quantification results were shown in the right panel.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/26/10.1182_blood-2011-05-354654/4/m_zh89991183460004.jpeg?Expires=1769113760&Signature=B6aaqCpx8fGN9Er-R7E1oF6bCdMHku3e~wrIFRilkInIP8SrV5vC1FpByv4~TG4L3avvr~ntZWpzQOjKe3ngrsLhskenKnSb-AvIEeykj04b9xdMnqgphyPKl1pord2nZ6fNbo-yPbU~opShQpRZZMbt9heN880rCllvAI-lpdYfvNvmhCmB~6AonO9Yfyhha2CP9QbV2J0Hdsv6QzA5DU3kkvJXMPeUcBzUlJhmkGYKvC1-B8u1Flx9iN3rh~R8fy-djQl3rCNHmFDYyesFD1~wB45xgCbhFLVUFIfnS4ya41TSk4ah4BjSQKvprBdeJoVN49UetPayQwA8CVXHRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal