Abstract
It has been reported that the intracellular antiapoptotic factor myeloid cell leukemia sequence 1 (Mcl-1) is required for mast cell survival in vitro, and that genetic manipulation of Mcl-1 can be used to delete individual hematopoietic cell populations in vivo. In the present study, we report the generation of C57BL/6 mice in which Cre recombinase is expressed under the control of a segment of the carboxypeptidase A3 (Cpa3) promoter. C57BL/6-Cpa3-Cre; Mcl-1fl/fl mice are severely deficient in mast cells (92%-100% reduced in various tissues analyzed) and also have a marked deficiency in basophils (58%-78% reduced in the compartments analyzed), whereas the numbers of other hematopoietic cell populations exhibit little or no changes. Moreover, Cpa3-Cre; Mcl-1fl/fl mice exhibited marked reductions in the tissue swelling and leukocyte infiltration that are associated with both mast cell- and IgE-dependent passive cutaneous anaphylaxis (except at sites engrafted with in vitro–derived mast cells) and a basophil- and IgE-dependent model of chronic allergic inflammation, and do not develop IgE-dependent passive systemic anaphylaxis. Our findings support the conclusion that Mcl-1 is required for normal mast cell and basophil development/survival in vivo in mice, and also suggest that Cpa3-Cre; Mcl-1fl/fl mice may be useful in analyzing the roles of mast cells and basophils in health and disease.
Introduction
Myeloid cell leukemia sequence 1 (Mcl-1)1 has been identified as an intracellular antiapoptotic factor in a variety of hematopoietic cells, both in vitro and in vivo.2-6 Human mast cells express Mcl-1,7,8 and Mcl-1 can promote the survival of some populations of human neoplastic mast cells in vitro.7 Basophils, granulocytes with many characteristics and functions that partially overlap with those of tissue mast cells,9-12 can also express Mcl-1.13 However, it is not clear to what extent Mcl-1 is important in the development and/or survival of mast cells or basophils in vivo.
Opferman et al showed that the genetic manipulation of Mcl-1 can be used to delete individual hematopoietic cell populations in mice.4 We therefore used this approach to examine the effects of reducing expression of Mcl-1 in the mast cell lineage in vivo. To attempt to delete Mcl-1 selectively in mast cells, we used the promoter for the peptidase carboxypeptidase A3 (CPA3; originally named mast cell carboxypeptidase A14 ). CPA3 is highly expressed in mast cells,15 but is also expressed in basophils16 and can be expressed in some populations of T-cell progenitors and thymic T cells17,18 and in certain hematopoietic progenitor cells.19
We generated C57BL/6 mice in which a segment of the Cpa3 promoter drives expression of Cre recombinase, and then mated these Cpa3-Cre transgenic mice to mice bearing a floxed allele of Mcl-1.4 We found that C57BL/6-Cpa3-Cre; Mcl-1fl/fl mice are severely deficient in mast cells and have a marked deficiency in basophils, and also exhibit striking impairment in mast cell- or basophil- and IgE-dependent biologic responses.
Methods
Mice
All animal experiments were carried out following protocols approved by the Stanford University Administrative Panel on Laboratory Animal Care. B6-Tg(Cpa3-cre)3Glli (Cpa3-Cre–transgenic mice) were generated by microinjecting the Cpa3-Cre transgene into embryonic stem cells in the B6 background (Stanford University). Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J(mT/mG) mice, obtained from The Jackson Laboratory, were crossed to Cpa3-Cre mice for Cre expression analysis. Mcl-1+/fl (B6;129-Mcl1tm3sjkJ) animals were obtained from The Jackson Laboratory. Mcl-1+/fl mice were bred to progeny from 2 Cpa3-Cre founder lines (founder lines #4 and #5) to obtain Cpa3-Cre; Mcl-1+/+, Cpa3-Cre; Mcl-1+/fl, and Cpa3-Cre; Mcl-1fl/fl animals, but only the Cpa3-Cre; Mcl-1fl/fl mice derived from founder line #4 exhibited a substantial mast cell deficiency. Therefore, the mice used were derived from crosses between founder line #4 (subsequently referred to as Cpa3-Cre mice) and Mcl-1fl animals, and these mice had been intercrossed a minimum of 6 generations into the C57BL/6 background. Heterozygous Cpa3-Cre mice were determined to have 5 copies of the Cpa3-Cre transgene by real-time PCR. To emphasize that Cpa3-Cre; Mcl-1fl/fl mice have deficiencies in mast cells and basophils that are independent of mutations affecting Kit, we call them informally in our laboratory “Hello Kitty” mast cell– and basophil-deficient mice. C57BL/6J (B6)-KitW-sh/W-sh mast cell–deficient mice and B6-Kit+/+ wild-type control mice were from stocks bred in our laboratory, as described previously.20 All experiments were performed at least 3 times unless otherwise indicated.
Abs, flow cytometry, and cell quantification
We used flow cytometry to identify and enumerate basophils (CD49b [DX5]+; FcϵRIα+) in the BM, peripheral blood, and spleen; mast cells (c-Kit+; FcϵRIα+) in peritoneal lavage fluid; B cells (B220+; CD3ϵ−) in peritoneal lavage fluid and spleen; eosinophils (CCR3+; SSChi) in the spleen and BM; macrophages/monocytes (F4/80+; Gr-1−) in BM, peritoneal lavage fluid, and spleen; neutrophils (Gr-1+) in BM, peritoneal lavage fluid, and spleen; T cells (CD3ϵ+; B220−) in the spleen and thymus; and erythroid cells (Ter119+) in the spleen. Briefly, RBCs were lysed with pH 7.3 ACK lysis buffer (0.15M NH4Cl, 1mM KHCO3, and 0.1mM EDTA, pH 8.0) for 5 minutes. Cells were blocked with unconjugated anti–CD16/CD32 on ice for 5 minutes and then stained with a combination of Abs on ice for 15 minutes. Abs used were: B220 (RA3-6B2; eBioscience), c-Kit (2B8; eBioscience), CCR3 (TG14/CCR3; BioLegend and R&D Systems), CD11b (M1/70; eBioscience), CD3ϵ (145-2C11; BioLegend), CD49b (DX5; BD Pharmingen and BioLegend), F4/80 (BM8; BioLegend), FcϵRIα (MAR-1; BioLegend and eBioscience), and Ter119 (TER-119; BioLegend). The expression of cell-surface markers was analyzed on a FACSAria II (BD Biosciences) using FlowJo Version 8.8.6 software (Stanford University and TreeStar). Dead cells (identified with propidium iodide; Invitrogen) were not included in the analysis. Blood lymphocytes, neutrophils, monocytes, eosinophils, erythrocytes, and platelets were counted using the Abbott Cell-Dyn 3500 automated hematology analyzer.
Histology
Some tissue specimens were fixed with 10% neutral buffered formalin and embedded in paraffin. Four-micrometer sections were stained with 0.1% Toluidine blue or H&E for histologic examination and enumeration of mast cells or leukocytes, respectively. To examine mast cell morphology in ears subjected to passive cutaneous anaphylaxis (PCA), specimens of ear pinnae were fixed in 2% paraformaldehyde, 2.5% glutaraldehyde, 0.1M cacodylate buffer, and 0.025% CaCl2, washed in 0.1M cacodylate buffer, and embedded in Epon (Electron Microscopy Sciences), and then 1-μm sections were stained with alkaline Giemsa (pH 8.2) as described previously.21 To examine mast cell numbers in mesenteric windows, samples of mesentery were spread on a glass slide, fixed in Carnoy solution and stained with Alcian blue/Safranin-O. For PCA and chronic allergic inflammation (CAI) experiments (see “PCA” and “CAI”), ear pinnae were collected 6 hours after challenge and fixed in 10% neutral buffered formalin for paraffin sections to be stained with H&E to enumerate leukocytes present in the ear dermis. In all histological assessments, cell numbers were enumerated by a single observer not aware of the identity (mouse group) of the individual sections. Cell numbers were based on counting 25 medium power fields (200×) for mast cells or high power fields (400×) for leukocytes from a minimum of 3 sections from individual tissues from each mouse, and the mean value was used as the number for that mouse. Mast cells were quantified according to area (per square millimeter) for all tissues except the forestomach, glandular stomach, duodenum, and ileum (per millimeter of mucosa and submucosa) and dorsal skin and ear pinna (per millimeter horizontal field length of dermis). Leukocytes also were quantified as per millimeter of horizontal field length of ear dermis. To visualize infiltrating basophils in CAI experiments, ear pinnae sections were pretreated as described previously,22 and incubated with anti–mMCP-8 (10 μg/mL) for 1 hour at room temperature, followed by HRP-conjugated goat anti–rat IgG (Santa Cruz Biotechnology) diluted 1:500. The sections were subsequently incubated in a DAB solution (Sigma-Aldrich) and counterstained with Giemsa. Images were captured with an Olympus BX60 microscope using a Retiga-2000R QImaging camera run by Image-Pro Plus Version 6.3 software (Media Cybernetics).
PCA
IgE-dependent PCA was induced in the ear pinna as described previously.23 Briefly, mice under isoflurane anesthesia were sensitized passively with IgE by intradermal injection of 20 ng of dinitrophenol (DNP)–specific IgE (α-DNP clone ϵ2624 ) kindly provided by Dr Fu-Tong Liu (University of California-Davis) in 20 μL of HMEM-Pipes buffer (Sigma-Aldrich) in the right ear pinna; mice received 20 μL of vehicle intradermally in the left ear pinna as a control. The next day, mice were challenged intravenously with 100 μg of DNP30-40–conjugated human serum albumin (DNP-HSA; Sigma-Aldrich) in 100 μL of NaCl. Immediately before and at intervals after antigen challenge, ear thickness was measured with a dial thickness gauge (G-1A; Ozaki). Mice were killed 6 hours after antigen challenge and ear pinnae were collected for histological analysis.
CAI
IgE-dependent CAI of the ear skin was induced as described previously.25 Briefly, mice under isoflurane anesthesia were sensitized IV with IgE (300 μg of trinitrophenol (TNP)–specific IgE mAb (IGELb4), kindly provided by Dr Hajime Karasuyama (Tokyo Medical and Dental University). A day later, 10 μg of TNP14-conjugated OVA (TNP-OVA; Biosearch Technologies) in 10 μL of PBS was injected intradermally with a microsyringe into the left ear of mice under isoflurane anesthesia, and an equal amount of unconjugated OVA (Sigma-Aldrich) was injected into the right ear as a control. Ear thickness was measured with a dial thickness gauge (G-1A; Ozaki) at the indicated time points.
PSA
IgE-dependent passive systemic anaphylaxis (PSA) was induced as described previously.26 Briefly, mice were sensitized passively with IgE by intraperitoneal injection of 20 ng of DNP-specific IgE in 100 μL of saline or were injected intraperitoneally with saline as a control and then challenged intraperitoneally the next day with 1 mg of DNP-HSA in 100 μL of saline. Immediately before and at intervals after antigen challenge, body temperature was measured with a rectal thermometer (Physitemp Instrument). Mice were killed 2 hours after antigen challenge.
Cell culture
Mouse femoral and tibial BM cells from C57BL/6J or various Cpa3-Cre mice were cultured for 6 weeks in 20% WEHI-3 cell-conditioned medium (as a source of IL-3) to generate BM-derived cultured mast cells (BMCMCs) or for 7-8 days to generate BM-derived basophils (BMBas). Mast cell differentiation was assessed by May-Grünwald-Giemsa staining of cytospin preparations for granule content and by flow cytometry for surface expression of c-Kit and FcϵRIα (all BMCMC preparations were > 95% pure). Basophil differentiation was assessed by flow cytometry for surface expression of DX5 and FcϵRIα.
Western blotting
BMCMCs were washed with DMEM and solubilized by boiling for 1 minute with Laemmli-SDS sample buffer (106 cells/50 μL). Total cell lysates were sheared (1 cc syringe; 26-ga needle), and then separated by SDS-PAGE, electroblotted onto PVDF membranes (Invitrogen), and probed with polyclonal Abs against Mcl-1 (Rockland Immunochemicals) or GAPDH (Fitzgerald Industries).
Statistics
Unless otherwise specified, data were examined for statistical significance using the Student t test (2-tailed, unpaired). We used 2-way ANOVA to compare time courses of responses. Significance was attributed when P ≤ .05 was observed.
Results
Mcl-1 is expressed by mouse mast cells in vitro
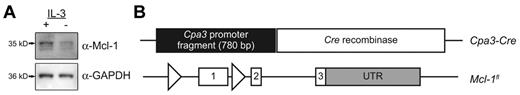
We first tested whether BMCMCs express the Mcl-1 protein in vitro. After 6 weeks of culture in IL-3–supplemented medium, BMCMCs expressed Mcl-1 protein, and the amount of Mcl-1 expression decreased when BMCMCs were maintained for 24 hours in the absence of IL-3 (Figure 1A). This observation agrees with previous findings that these culture conditions result in decreased levels of Bcl-2 family antiapoptotic proteins in BMCMCs, which is concomitant with a loss of mast cell viability.27
Mcl-1 expressed by mast cells in vitro and maps of the Cpa3-Cre and Mcl-1fl transgenes. (A) Mcl-1 is expressed by mast cells in vitro. BMCMCs (5 × 105) from male wild-type C57BL/6J mice were subjected to electrophoresis and Western blotting using a polyclonal Ab against Mcl-1 (Rockland) and reprobed with anti-GAPDH Ab to show loading. Bands represent the long (35 kDa) and short (32 kDa) forms of Mcl-1. BMCMCs were cultured for 24 hours with 10% FCS alone (IL-3−) or FCS + IL-3 (IL-3+; 20% Wehi-3–conditioned medium). Expression of Mcl-1 decreased after 24 hours of culture in medium without added IL-3. Results are representative of those obtained in 6 independent experiments. (B) Map of the Cpa3-Cre transgene. A 780-bp fragment of the region just 5′ to the Cpa3 transcription start site was used to drive expression of Cre recombinase in vivo (Cpa3-Cre). These transgenic mice were bred with mice in which the first exon of Mcl-14 has been flanked by loxP sites (triangles).
Mcl-1 expressed by mast cells in vitro and maps of the Cpa3-Cre and Mcl-1fl transgenes. (A) Mcl-1 is expressed by mast cells in vitro. BMCMCs (5 × 105) from male wild-type C57BL/6J mice were subjected to electrophoresis and Western blotting using a polyclonal Ab against Mcl-1 (Rockland) and reprobed with anti-GAPDH Ab to show loading. Bands represent the long (35 kDa) and short (32 kDa) forms of Mcl-1. BMCMCs were cultured for 24 hours with 10% FCS alone (IL-3−) or FCS + IL-3 (IL-3+; 20% Wehi-3–conditioned medium). Expression of Mcl-1 decreased after 24 hours of culture in medium without added IL-3. Results are representative of those obtained in 6 independent experiments. (B) Map of the Cpa3-Cre transgene. A 780-bp fragment of the region just 5′ to the Cpa3 transcription start site was used to drive expression of Cre recombinase in vivo (Cpa3-Cre). These transgenic mice were bred with mice in which the first exon of Mcl-14 has been flanked by loxP sites (triangles).
The Cpa3 promoter can be used to express Cre recombinase in mast cells and basophils in vivo
Based on our in vitro findings, we hypothesized that it might be possible to reduce mast cell numbers in vivo by conditionally deleting Mcl-1 expression in these cells. To target mast cells, we constructed transgenic mice that express Cre recombinase under the control of a 780-bp fragment of the Cpa3 gene (Cpa3-Cre) (Figure 1B). This region, which is just 5′ to the start codon of Cpa3, contains GATA-binding domains28 thought to regulate Cpa3 transcription at early stages of mast cell differentiation.29
To examine the extent of Cre expression in various hematopoietic lineages in Cpa3-Cre mice, we crossed these mice to mT/mG reporter mice30 ; the latter mice express membrane-targeted red fluorescence in all cells except those in which Cre-mediated excision of the mT sequence (that drives expression of the membrane-targeted tandem dimer Tomato), and subsequent expression of mG (membrane-targeted enhanced green fluorescent protein [GFP]), results in green rather than red fluorescence. This analysis indicated that Cpa3-Cre; mT/mG mice exhibited high levels of Cre expression in peritoneal mast cells (Figure 2A). In contrast, we detected no evidence of Cre expression in Cpa3-Cre; mT/mG mice in peritoneal macrophages (Figure 2B), but a small population of splenic erythroid cells were GFP+ (Figure 2C). Voehringer et al identified Cpa3 as a gene that is also highly expressed in mouse basophils,16 and we detected Cre expression in the spleen basophils of Cpa3-Cre; mT/mG mice (Figure 2D). However, similar percentages of splenic eosinophils (Figure 2E) and neutrophils (Figure 2F) also appeared to express Cre, and for all 3 granulocyte populations, the GFP+ and GFP− cells from the spleen or BM (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) expressed very similar levels of cell-specific markers and forward versus side scatter. Small numbers of thymic and splenic T cells expressed mG in Cpa3-Cre; mT/mG mice (Figure 2G-H), a finding in agreement with reports indicating that Cpa3 can be expressed in T-cell populations in mice17,18 ; in contrast, splenic B cells were essentially negative (Figure 2I).
The Cpa3 promoter drives Cre expression in mast cells and basophils in vivo. (A-I) Expression of Cre in Cpa3-Cre mice, representative of similar results that were obtained in the 3 independent experiments performed. Fluorescence intensity plots depict GFP in cells from Cpa3-Cre; mT/mG mice (green solid lines) that express Cre, but not in mT/mG–only mice (red dashed lines) or Cpa3-Cre–only mice (gray filled curves). Percentage of GFP+ cells are indicated on the plots for Cpa3-Cre; mT/mG mice for each of the following cell populations: (A) peritoneal mast cells, (B) peritoneal macrophages, (C) splenic Ter119+ (erythroid) cells, (D) splenic basophils, (E) splenic eosinophils, (F) splenic neutrophils, (G) thymic T cells, (H) splenic T cells, and (I) splenic B cells.
The Cpa3 promoter drives Cre expression in mast cells and basophils in vivo. (A-I) Expression of Cre in Cpa3-Cre mice, representative of similar results that were obtained in the 3 independent experiments performed. Fluorescence intensity plots depict GFP in cells from Cpa3-Cre; mT/mG mice (green solid lines) that express Cre, but not in mT/mG–only mice (red dashed lines) or Cpa3-Cre–only mice (gray filled curves). Percentage of GFP+ cells are indicated on the plots for Cpa3-Cre; mT/mG mice for each of the following cell populations: (A) peritoneal mast cells, (B) peritoneal macrophages, (C) splenic Ter119+ (erythroid) cells, (D) splenic basophils, (E) splenic eosinophils, (F) splenic neutrophils, (G) thymic T cells, (H) splenic T cells, and (I) splenic B cells.
Evidence that Mcl-1 is a survival factor for mast cells and basophils in vitro and in vivo
Our results with the Cpa3-Cre; mT/mG reporter mice indicated that levels of Cpa3 that were high enough to drive substantial Cpa3-Cre–dependent loss of red fluorescence were restricted predominantly to mast cells, whereas lesser amounts of Cpa3-Cre–dependent loss of red fluorescence were detected in all 3 granulocyte populations. To test the extent to which mast cells and various granulocytes require Mcl-1 for development and/or survival, we crossed mice carrying the Cpa3-Cre transgene with mice in which the first exon of Mcl-1 is flanked by LoxP sites (Mcl-1fl)4 (Figure 1B). Because male mice carrying 2 floxed Mcl-1 alleles exhibit reduced fertility (The Jackson Laboratory), heterozygous males (Mcl-1+/fl) were crossed with either heterozygous (Mcl-1+/fl) or homozygous (Mcl-1fl/fl) females to generate mice for subsequent cell lineage and functional analyses.
Cpa3-Cre; Mcl-1fl/fl mice appeared to be grossly normal, based on both external inspection and autopsy examination of their organs. However, Cpa3-Cre; Mcl-1fl/fl mice exhibited dramatic reductions in mast cell numbers in nearly all tissues and anatomic sites examined (Figure 3 and supplemental Figure 2). In control Cpa3-Cre; Mcl-1+/+ mice, mast cells were present in numbers very similar to those in wild-type C57BL/6J mice (eg, see Grimbaldeston et al31 ). In contrast, 6-week- to 10-month-old Cpa3-Cre; Mcl-1fl/fl mice exhibited reductions in mast cell numbers that ranged from 92%-100% in all sites examined except for the spleen, where no differences were detected in the small numbers of mast cells (Figure 3B-E and supplemental Figure 2). We found that the peritoneal mast cells detected in Cpa3-Cre; Mcl-1fl/fl mice exhibited levels of surface expression of FcϵRI that were similar to those in the peritoneal mast cells of control Cpa3-Cre; Mcl-1+/+ mice (Figure 3E). Cpa3-Cre; Mcl-1fl/+ mouse tissues were also assessed for mast cell numbers, but no significant differences were found between the corresponding mast cell populations in mice with 1 versus 2 functional copies of Mcl-1 (data not shown).
Cpa3-Cre; Mcl-1fl/fl mice have markedly reduced numbers of mast cells. (A) Toluidine blue staining for mast cells in 4-μm-thick paraffin sections of glandular stomach wall (top) and dorsal skin (bottom) in Cpa3-Cre; Mcl-1+/+ mice (left) and Cpa3-Cre; Mcl-1fl/fl mice (right) shows a marked reduction in numbers of mast cells (purple) in mice in which Mcl-1 has been selectively deleted by Cpa3-Cre. Scale bar indicates 100 μm. (B-C) Numbers of mast cells in various tissues in Cpa3-Cre; Mcl-1+/+ (control) mice or Cpa3-Cre; Mcl-1fl/fl mice. Numbers of mast cells are shown as means + SEM per millimeter or per square millimeter of 4-μm-thick paraffin sections stained with 0.1% Toluidine blue (see “Methods”) in tissues from Cpa3-Cre; Mcl-1+/+ (n = 16) or Cpa3-Cre; Mcl-1fl/fl (n = 14) mice, except for mesentery window (n = 12 per group) and mammary stroma (n = 6 per group). (D) Percentage of mast cells in the live-cell population isolated from peritoneal lavage fluid from Cpa3-Cre; Mcl-1+/+ (control, n = 10) or Cpa3-Cre; Mcl-1fl/fl (n = 10) mice, analyzed by flow cytometry (FcϵRIα+; c-Kit+). Peritoneal mast cell numbers are shown as means + SEM. (B-D) **P < .01 and ***P < .001 versus corresponding values for Cpa3-Cre; Mcl-1+/+ mice. (E) Representative flow cytometry plots show comparable expression of FcϵRIα+ on peritoneal mast cells isolated from Cpa3-Cre; Mcl-1+/+ or Cpa3-Cre; Mcl-1fl/fl mice. In panels B-D, mice ranged from 3-10 months of age; mouse age did not influence mast cell numbers in each genotype (data not shown). Images were captured with an Olympus BX60 microscope with a 20× objective using a Retiga-2000R QImaging camera run by Image-Pro Plus Version 6.3 software (Media Cybernetics) and exported into Adobe Photoshop (CS3), in which images were white balanced and resized.
Cpa3-Cre; Mcl-1fl/fl mice have markedly reduced numbers of mast cells. (A) Toluidine blue staining for mast cells in 4-μm-thick paraffin sections of glandular stomach wall (top) and dorsal skin (bottom) in Cpa3-Cre; Mcl-1+/+ mice (left) and Cpa3-Cre; Mcl-1fl/fl mice (right) shows a marked reduction in numbers of mast cells (purple) in mice in which Mcl-1 has been selectively deleted by Cpa3-Cre. Scale bar indicates 100 μm. (B-C) Numbers of mast cells in various tissues in Cpa3-Cre; Mcl-1+/+ (control) mice or Cpa3-Cre; Mcl-1fl/fl mice. Numbers of mast cells are shown as means + SEM per millimeter or per square millimeter of 4-μm-thick paraffin sections stained with 0.1% Toluidine blue (see “Methods”) in tissues from Cpa3-Cre; Mcl-1+/+ (n = 16) or Cpa3-Cre; Mcl-1fl/fl (n = 14) mice, except for mesentery window (n = 12 per group) and mammary stroma (n = 6 per group). (D) Percentage of mast cells in the live-cell population isolated from peritoneal lavage fluid from Cpa3-Cre; Mcl-1+/+ (control, n = 10) or Cpa3-Cre; Mcl-1fl/fl (n = 10) mice, analyzed by flow cytometry (FcϵRIα+; c-Kit+). Peritoneal mast cell numbers are shown as means + SEM. (B-D) **P < .01 and ***P < .001 versus corresponding values for Cpa3-Cre; Mcl-1+/+ mice. (E) Representative flow cytometry plots show comparable expression of FcϵRIα+ on peritoneal mast cells isolated from Cpa3-Cre; Mcl-1+/+ or Cpa3-Cre; Mcl-1fl/fl mice. In panels B-D, mice ranged from 3-10 months of age; mouse age did not influence mast cell numbers in each genotype (data not shown). Images were captured with an Olympus BX60 microscope with a 20× objective using a Retiga-2000R QImaging camera run by Image-Pro Plus Version 6.3 software (Media Cybernetics) and exported into Adobe Photoshop (CS3), in which images were white balanced and resized.
In vitro evidence suggests that the few mast cells that persist in Cpa3-Cre; Mcl-1fl/fl mice may be those that have the highest levels of Mcl-1. Specifically, when we examined the generation of mast cells from adult BM cells derived from Mcl-1fl/fl versus Cpa3-Cre; Mcl-1fl/fl mice, we found that BM cells from Cpa3-Cre; Mcl-1fl/fl mice generated far fewer BMCMCs than did those from Mcl-1fl/fl mice, if any survived at all (supplemental Figure 3A). However, those rare mast cells that persisted in the cultures of Cpa3-Cre; Mcl-1fl/fl mouse BM cells had levels of Mcl-1 protein that were similar to those of the corresponding Mcl-1fl/fl cells (supplemental Figure 3B), suggesting that these persisting Cpa3-Cre; Mcl-1fl/fl cells expressed sufficient Mcl-1 protein to evade death even after 6 weeks of in vitro culture. The Mcl-1 expression in such cells may be the result of incomplete excision of the first exon of Mcl-1 by Cpa3-driven Cre in these mast cells.
Cpa3-Cre; Mcl-1fl/fl mice exhibit a marked reduction in basophils
Our findings in Cpa3-Cre; mT/mG reporter mice suggested that basophils, eosinophils, and neutrophils also may be affected in Cpa3-Cre; Mcl-1fl/fl mice. We therefore examined whether numbers of various hematopoietic cells are reduced in Cpa3-Cre; Mcl-1fl/fl mice. We observed substantial—and statistically significant—reductions in basophil numbers in the spleen, BM, and blood of Cpa3-Cre; Mcl-1fl/fl versus control Cpa3-Cre; Mcl-1+/+ mice (Figure 4A). In 3- to 10-month-old mice, basophil numbers were reduced 58% in the spleen, 78% in the BM, and 74% in the blood. The basophils detected in Cpa3-Cre; Mcl-1fl/fl mice exhibited levels of surface expression of FcϵRI that were similar to those in the basophils of the control Cpa3-Cre; Mcl-1+/+ mice (Figure 4A). Cpa3-Cre; Mcl-1fl/fl mouse BM yielded on average 43.3% fewer BMBas after 8 days of in vitro culture than did Cpa3-Cre; Mcl-1+/+ BM cells. In the 3 independent experiments performed, each with cells from n = 3 mice/genotype, the yield of BMBas from Cpa3-Cre; Mcl-1fl/fl mouse BM cells was reduced compared with that from Cpa3-Cre; Mcl-1+/+ cells by 31.2%, 45.9%, and 52.8%, with the differences between numbers of BMBas in the 2 groups in these 3 experiments at P = .052, P = .007, and P = .007, respectively, by Student unpaired 2-tailed t test.
Cpa3-Cre; Mcl-1fl/fl mice have reduced numbers of basophils but not other leukocytes. (A-F) Percentages (A-C) or absolute numbers (D-F) of leukocytes (A-E) or platelets and erythrocytes (F) are depicted as values from individual mice, with bars indicating means ± SEM. *P < .05 and ***P < .001 versus corresponding values for Cpa3-Cre; Mcl-1+/+ mice (controls). All other comparisons are not significant (P > .05). (A) Left: Percentage of basophils (FcϵRIα+; DX5+; c-Kit−) in the live-cell population isolated from Cpa3-Cre mouse spleen (Mcl-1+/+, n = 6; Mcl-1fl/fl, n = 6), BM (Mcl-1+/+, n = 9; Mcl-1fl/fl, n = 8), or blood (Mcl-1+/+, n = 8; Mcl-1fl/fl, n = 8), as analyzed by flow cytometry. Right: Representative flow cytometry plots show comparable expression of FcϵRIα+ on basophils isolated from Cpa3-Cre; Mcl-1+/+ or Cpa3-Cre; Mcl-1fl/fl mouse blood. (B) Percentage of neutrophils (Gr-1+) in the live-cell population isolated from Cpa3-Cre mouse spleen, BM, or peritoneal lavage fluid (Mcl-1+/+, n = 11; Mcl-1fl/fl, n = 10), as analyzed by flow cytometry. (C) Percentage of T cells (CD3+) in the live-cell population isolated from Cpa3-Cre mouse spleen (Mcl-1+/+, n = 8; Mcl-1fl/fl, n = 7) or thymus (Mcl-1+/+, n = 6; Mcl-1fl/fl, n = 6), as analyzed by flow cytometry. (D) Numbers of lymphocytes and neutrophils in blood from Cpa3-Cre mice (Mcl-1+/+, n = 11; Mcl-1fl/fl, n = 10). (E) Numbers of monocytes (Mcl-1+/+, n = 8; Mcl-1fl/fl, n = 8) and eosinophils (Mcl-1+/+, n = 4; Mcl-1fl/fl, n = 7) in blood from Cpa3-Cre mice. (F) Numbers of platelets and erythrocytes in blood from Cpa3-Cre mice (Mcl-1+/+, n = 11; Mcl-1fl/fl, n = 10).
Cpa3-Cre; Mcl-1fl/fl mice have reduced numbers of basophils but not other leukocytes. (A-F) Percentages (A-C) or absolute numbers (D-F) of leukocytes (A-E) or platelets and erythrocytes (F) are depicted as values from individual mice, with bars indicating means ± SEM. *P < .05 and ***P < .001 versus corresponding values for Cpa3-Cre; Mcl-1+/+ mice (controls). All other comparisons are not significant (P > .05). (A) Left: Percentage of basophils (FcϵRIα+; DX5+; c-Kit−) in the live-cell population isolated from Cpa3-Cre mouse spleen (Mcl-1+/+, n = 6; Mcl-1fl/fl, n = 6), BM (Mcl-1+/+, n = 9; Mcl-1fl/fl, n = 8), or blood (Mcl-1+/+, n = 8; Mcl-1fl/fl, n = 8), as analyzed by flow cytometry. Right: Representative flow cytometry plots show comparable expression of FcϵRIα+ on basophils isolated from Cpa3-Cre; Mcl-1+/+ or Cpa3-Cre; Mcl-1fl/fl mouse blood. (B) Percentage of neutrophils (Gr-1+) in the live-cell population isolated from Cpa3-Cre mouse spleen, BM, or peritoneal lavage fluid (Mcl-1+/+, n = 11; Mcl-1fl/fl, n = 10), as analyzed by flow cytometry. (C) Percentage of T cells (CD3+) in the live-cell population isolated from Cpa3-Cre mouse spleen (Mcl-1+/+, n = 8; Mcl-1fl/fl, n = 7) or thymus (Mcl-1+/+, n = 6; Mcl-1fl/fl, n = 6), as analyzed by flow cytometry. (D) Numbers of lymphocytes and neutrophils in blood from Cpa3-Cre mice (Mcl-1+/+, n = 11; Mcl-1fl/fl, n = 10). (E) Numbers of monocytes (Mcl-1+/+, n = 8; Mcl-1fl/fl, n = 8) and eosinophils (Mcl-1+/+, n = 4; Mcl-1fl/fl, n = 7) in blood from Cpa3-Cre mice. (F) Numbers of platelets and erythrocytes in blood from Cpa3-Cre mice (Mcl-1+/+, n = 11; Mcl-1fl/fl, n = 10).
However, the Cpa3-Cre; Mcl-1fl/fl mice exhibited no significant alterations compared with values in control mice in any of the other leukocyte populations tested (Figure 4 and supplemental Figure 4), including eosinophils in the blood, spleen, and BM (Figure 4E and supplemental Figure 4A) and neutrophils in the blood, BM, and peritoneal cavity (Figure 4B,D), except for a modest (56%) increase in numbers of neutrophils in the spleen (Figure 4B). We did not detect significant changes in numbers of T cells (Figure 4C) or B cells (supplemental Figure 4C) in Cpa3-Cre; Mcl-1fl/fl mice or in the absolute numbers of lymphocytes or myeloid cells analyzed in the blood (Figure 4D-E) or in the spleen, BM, or peritoneal fluid (supplemental Figure 4A-B). Although Cpa3-Cre; Mcl-1fl/fl mice had normal numbers of platelets (Figure 4F), they had a mild macrocytic anemia (Figure 4F and supplemental Figure 5), with an approximately 31% reduction in numbers of blood erythrocytes (Figure 4F) and a 24% reduction in hematocrit (supplemental Figure 5) compared with Cpa3-Cre; Mcl-1+/+ control mice. Whereas the physiologic significance of such a mild anemia is not clear, this observation does support recent findings suggesting that mast cell and megakaryocyte/erythroid progenitors may be more closely linked than was realized previously.19
Cpa3-Cre; Mcl-1fl/fl mice exhibit markedly reduced mast cell–dependent tissue swelling and leukocyte recruitment in IgE-dependent PCA. The data shown in panels A through C were pooled from the 3 independent experiments performed, each of which gave similar results. (A) Cpa3-Cre; Mcl-1fl/fl mice (n = 9) and Cpa3-Cre; Mcl-1+/+ control mice (n = 12) were sensitized by intradermal injection of 20 ng of anti-DNP IgE into the right ear pinna, with vehicle injection into the left ear pinna as a control. Mice were challenged by retro-orbital injection of 100 μg of DNP-HSA the next day. Ear swelling was measured at the indicated time points and data are shown as means ± SEM. **P < .01 and ***P < .001 versus corresponding values for Cpa3-Cre; Mcl-1+/+ mice at the indicated time points. +P < .05, ++P < .01, and +++P < .001 versus corresponding values for contralateral vehicle control ears in mice of the same genotype at the indicated time points. P < .0001 by 2-way ANOVA for the swelling response in ears sensitized with anti-DNP IgE in Cpa3-Cre; Mcl-1fl/fl versus Cpa3-Cre; Mcl-1+/+mice. (B-C) Numbers of mast cells (B) and leukocytes (C) in the dermis of ear pinnae of Cpa3-Cre; Mcl-1fl/fl mice (n = 9) and Cpa3-Cre; Mcl-1+/+ control mice (n = 12) 6 hours after induction of PCA (IgE) or after in vehicle-treated control ears (vehicle). Data are shown as means + SEM for mast cells or leukocytes per millimeter of dermis. ***P < .001 versus corresponding values for Cpa3-Cre; Mcl-1+/+ mice; +++P < .001 versus corresponding values for vehicle-treated mice in mice of the same genotype. (D) Giemsa staining to demonstrate mast cells (top, in 1-μm-thick, Epon-embedded sections) and H&E staining to demonstrate leukocytes (bottom, in 4-μm-thick, paraffin-embedded sections) in ear pinnae 6 hours after induction of PCA reactions. There are decreased numbers of mast cells (solid arrowheads in upper panels) and leukocytes (some indicated by solid arrows in lower panels) in sections from Cpa3-Cre; Mcl-1fl/fl mice (right panels) versus Cpa3-Cre; Mcl-1+/+ mice (left panels) subjected to PCA. Top: Some mast cells (*) exhibit alterations of the staining or location of many cytoplasmic granules, changes that are indicative of degranulation, whereas other mast cells (+) exhibit few or no granules exhibiting such changes. Bottom: Black arrows indicate leukocytes (primarily neutrophils). Insets depict selected mast cells exhibiting morphological evidence of extensive (*) or minimal or no (+) degranulation. Scale bars indicate 50 μm (for insets, scale bars indicate 5 μm); e indicates epidermis. Images were captured with an Olympus BX60 microscope with a 20× objective using a Retiga-2000R QImaging camera run by Image-Pro Plus Version 6.3 software (Media Cybernetics) and exported into Adobe Photoshop (CS3), in which images were white balanced and resized.
Cpa3-Cre; Mcl-1fl/fl mice exhibit markedly reduced mast cell–dependent tissue swelling and leukocyte recruitment in IgE-dependent PCA. The data shown in panels A through C were pooled from the 3 independent experiments performed, each of which gave similar results. (A) Cpa3-Cre; Mcl-1fl/fl mice (n = 9) and Cpa3-Cre; Mcl-1+/+ control mice (n = 12) were sensitized by intradermal injection of 20 ng of anti-DNP IgE into the right ear pinna, with vehicle injection into the left ear pinna as a control. Mice were challenged by retro-orbital injection of 100 μg of DNP-HSA the next day. Ear swelling was measured at the indicated time points and data are shown as means ± SEM. **P < .01 and ***P < .001 versus corresponding values for Cpa3-Cre; Mcl-1+/+ mice at the indicated time points. +P < .05, ++P < .01, and +++P < .001 versus corresponding values for contralateral vehicle control ears in mice of the same genotype at the indicated time points. P < .0001 by 2-way ANOVA for the swelling response in ears sensitized with anti-DNP IgE in Cpa3-Cre; Mcl-1fl/fl versus Cpa3-Cre; Mcl-1+/+mice. (B-C) Numbers of mast cells (B) and leukocytes (C) in the dermis of ear pinnae of Cpa3-Cre; Mcl-1fl/fl mice (n = 9) and Cpa3-Cre; Mcl-1+/+ control mice (n = 12) 6 hours after induction of PCA (IgE) or after in vehicle-treated control ears (vehicle). Data are shown as means + SEM for mast cells or leukocytes per millimeter of dermis. ***P < .001 versus corresponding values for Cpa3-Cre; Mcl-1+/+ mice; +++P < .001 versus corresponding values for vehicle-treated mice in mice of the same genotype. (D) Giemsa staining to demonstrate mast cells (top, in 1-μm-thick, Epon-embedded sections) and H&E staining to demonstrate leukocytes (bottom, in 4-μm-thick, paraffin-embedded sections) in ear pinnae 6 hours after induction of PCA reactions. There are decreased numbers of mast cells (solid arrowheads in upper panels) and leukocytes (some indicated by solid arrows in lower panels) in sections from Cpa3-Cre; Mcl-1fl/fl mice (right panels) versus Cpa3-Cre; Mcl-1+/+ mice (left panels) subjected to PCA. Top: Some mast cells (*) exhibit alterations of the staining or location of many cytoplasmic granules, changes that are indicative of degranulation, whereas other mast cells (+) exhibit few or no granules exhibiting such changes. Bottom: Black arrows indicate leukocytes (primarily neutrophils). Insets depict selected mast cells exhibiting morphological evidence of extensive (*) or minimal or no (+) degranulation. Scale bars indicate 50 μm (for insets, scale bars indicate 5 μm); e indicates epidermis. Images were captured with an Olympus BX60 microscope with a 20× objective using a Retiga-2000R QImaging camera run by Image-Pro Plus Version 6.3 software (Media Cybernetics) and exported into Adobe Photoshop (CS3), in which images were white balanced and resized.
Cpa3-Cre; Mcl-1fl/fl mice exhibit markedly reduced PCA
To determine whether the marked deficiency in mast cells in Cpa3-Cre; Mcl-1fl/fl mice resulted in reduced mast cell function in vivo, we examined a classic example of a mast cell–dependent biologic response, IgE-dependent PCA.23 Cpa3-Cre; Mcl-1+/+ mice developed a robust local inflammatory response, with tissue swelling that peaked approximately 30 minutes after antigen challenge (Figure 5A). In contrast, the tissue swelling was markedly reduced in identically treated Cpa3-Cre; Mcl-1fl/fl mice (Figure 5A).
As expected, mast cell numbers in the ear pinnae dermis of Cpa3-Cre; Mcl-1fl/fl mice were very low compared with those in Cpa3-Cre; Mcl-1+/+ mice, and were not significantly different in IgE- versus vehicle-treated ears 6 hours after antigen challenge (Figure 5B and 5D). In addition to having much higher numbers of dermal mast cells, Cpa3-Cre; Mcl-1+/+ control mice exhibited a reduction in mast cell numbers in ear pinnae at sites of PCA reactions versus sites of vehicle injection (Figure 5B), probably because of the difficulty in identifying extensively degranulated mast cells using a counting method that requires identification of the cells' cytoplasmic granules (Figure 5D top left). Some of the rare mast cells observed in the dermis of specimens from Cpa3-Cre; Mcl-1fl/fl mice also exhibited histological evidence of degranulation (Figure 5D top right). This finding suggests that activation of the rare mast cells present in the skin of Cpa3-Cre; Mcl-1fl/fl mice by IgE and specific antigen probably accounted for the weak tissue-swelling response detected in these animals (Figure 5A). However, at 6 hours after antigen challenge, there was no significant difference in the numbers of leukocytes present in the IgE- versus vehicle-injected ear pinnae of Cpa3-Cre; Mcl-1fl/fl mice, whereas the numbers of leukocytes (mainly consisting of neutrophils) were markedly increased in the dermis 6 hours after antigen challenge in IgE-dependent PCA reactions in Cpa3-Cre; Mcl-1+/+ control mice (Figure 5C-D).
These results clearly show that Cpa3-Cre; Mcl-1fl/fl mice lack sufficient mast cells to orchestrate a robust IgE-dependent PCA response in vivo. Indeed, even though the few mast cells present in the ear dermis of these mice may have accounted for the slight and quickly resolved ear swelling observed in such animals (and the attenuated swelling response depicted in Figure 5A reflected mainly the responses which occurred in the 2 Cpa3-Cre; Mcl-1fl/fl mice with the highest numbers of ear mast cells), the Cpa3-Cre; Mcl-1fl/fl mice exhibited no detectable IgE-dependent increase in dermal leukocytes by 6 hours after antigen challenge (Figure 5C). In contrast, both increased tissue swelling (associated with local extravasation of Evan blue dye) and enhanced leukocyte recruitment occurred at PCA reaction sites elicited in the pinnae of Cpa3-Cre; Mcl-1fl/fl mice that had been engrafted intradermally with mast cells 8 weeks earlier (supplemental Figure 6A-C and 6E-F). Therefore, Cpa3-Cre–mediated deletion of Mcl-1 results in a skin mast cell deficiency that largely eliminates the ability of such mice to develop tissue swelling or leukocyte infiltration at sites challenged to express IgE- and antigen-dependent PCA reactions, but the ability to express IgE-dependent PCA is enhanced when such mice undergo local engraftment with mast cells. Cpa3-Cre; Mcl-1fl/fl mice may also be engrafted with BMCMCs in the peritoneal cavity (supplemental Figure 6D).
Cpa3-Cre; Mcl-1fl/fl mice have markedly reduced basophil-dependent tissue swelling and leukocyte recruitment in IgE-dependent CAI. (A) Cpa3-Cre; Mcl-1fl/fl mice (n = 10), and Cpa3-Cre; Mcl-1+/+ control mice (n = 14) were sensitized passively by IV injection of 300 μg of IgE anti-TNP. Mice were challenged the next day by intradermal injection of 10 μg of TNP-OVA into the left ear pinna and OVA (as a control) into the right ear pinna. Ear swelling was measured daily and the results are shown as means ± SEM. The data shown were pooled from the 3 independent experiments performed, each of which gave similar results. *P < .05; **P < .01; and ***P < .001 comparing swelling in ears of IgE-treated Cpa3-Cre; Mcl-1fl/fl mice versus corresponding values for Cpa3-Cre; Mcl-1+/+mice at the indicated time points. P < .0001 by 2-way ANOVA comparing the ear swelling responses in IgE-injected Cpa3-Cre; Mcl-1fl/fl versus Cpa3-Cre; Mcl-1+/+ mice. (B) Immunohistochemical visualization of basophils by staining with an anti-mMCP8 Ab (DAB substrate) and Giemsa counterstaining (top panel, in 4-μm-thick, paraffin-embedded sections) and H&E staining to demonstrate leukocytes (bottom panel, in 4-μm-thick, paraffin-embedded sections) in ear pinnae 3 days after induction of CAI reactions. There are markedly decreased numbers of basophils (brown) and leukocytes in sections from Cpa3-Cre; Mcl-1fl/fl mice (right) versus Cpa3-Cre; Mcl-1+/+ control mice (left) subjected to CAI. Scale bars indicate 100 μm (for insets, scale bars indicate 25 μm); *, ear cartilage; e, epidermis. Images were captured with an Olympus BX60 microscope with a 20× objective using a Retiga-2000R QImaging camera run by Image-Pro Plus Version 6.3 software (Media Cybernetics) and exported into Adobe Photoshop (CS3), in which images were white balanced and resized.
Cpa3-Cre; Mcl-1fl/fl mice have markedly reduced basophil-dependent tissue swelling and leukocyte recruitment in IgE-dependent CAI. (A) Cpa3-Cre; Mcl-1fl/fl mice (n = 10), and Cpa3-Cre; Mcl-1+/+ control mice (n = 14) were sensitized passively by IV injection of 300 μg of IgE anti-TNP. Mice were challenged the next day by intradermal injection of 10 μg of TNP-OVA into the left ear pinna and OVA (as a control) into the right ear pinna. Ear swelling was measured daily and the results are shown as means ± SEM. The data shown were pooled from the 3 independent experiments performed, each of which gave similar results. *P < .05; **P < .01; and ***P < .001 comparing swelling in ears of IgE-treated Cpa3-Cre; Mcl-1fl/fl mice versus corresponding values for Cpa3-Cre; Mcl-1+/+mice at the indicated time points. P < .0001 by 2-way ANOVA comparing the ear swelling responses in IgE-injected Cpa3-Cre; Mcl-1fl/fl versus Cpa3-Cre; Mcl-1+/+ mice. (B) Immunohistochemical visualization of basophils by staining with an anti-mMCP8 Ab (DAB substrate) and Giemsa counterstaining (top panel, in 4-μm-thick, paraffin-embedded sections) and H&E staining to demonstrate leukocytes (bottom panel, in 4-μm-thick, paraffin-embedded sections) in ear pinnae 3 days after induction of CAI reactions. There are markedly decreased numbers of basophils (brown) and leukocytes in sections from Cpa3-Cre; Mcl-1fl/fl mice (right) versus Cpa3-Cre; Mcl-1+/+ control mice (left) subjected to CAI. Scale bars indicate 100 μm (for insets, scale bars indicate 25 μm); *, ear cartilage; e, epidermis. Images were captured with an Olympus BX60 microscope with a 20× objective using a Retiga-2000R QImaging camera run by Image-Pro Plus Version 6.3 software (Media Cybernetics) and exported into Adobe Photoshop (CS3), in which images were white balanced and resized.
Cpa3-Cre; Mcl-1fl/fl mice exhibit markedly reduced IgE-dependent CAI
In light of the 74% reduction in blood basophils in Cpa3-Cre; Mcl-1fl/fl mice (Figure 4A), we investigated whether Cpa3-Cre; Mcl-1fl/fl mice exhibited attenuation of a basophil-dependent biologic response, namely IgE-dependent CAI of the skin.25 We found that the tissue swelling associated with the IgE-dependent CAI response was essentially eliminated in Cpa3-Cre; Mcl-1fl/fl mice (Figure 6A). Leukocyte numbers (mainly consisting of neutrophils and eosinophils, but with a prominent infiltrate of basophils) were noticeably increased in the dermis 3 days after antigen challenge in IgE-dependent CAI reactions in Cpa3-Cre; Mcl-1+/+ control mice, whereas Cpa3-Cre; Mcl-1fl/fl mice virtually lacked leukocyte infiltration and exhibited essentially no mMCP8+ basophils by immunohistochemistry (Figure 6B).
Cpa3-Cre; Mcl-1fl/fl mice exhibit markedly reduced PSA
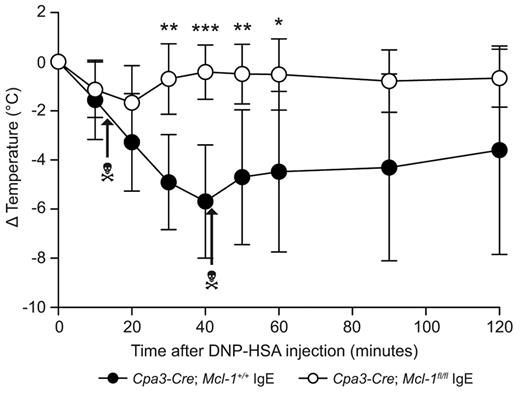
We assessed an IgE-dependent systemic immune response in Cpa3-Cre; Mcl-1fl/fl mice by examining a model of IgE-dependent PSA. In IgE-sensitized Cpa3-Cre; Mcl-1+/+ mice, injection of antigen resulted in marked hypothermia and, in some mice, death (Figure 7). In contrast, IgE-sensitized Mcl-1fl/fl mice were essentially unreactive to identical antigen challenge, and none of the mice died (Figure 7).
IgE-dependent PSA is markedly reduced in Cpa3-Cre; Mcl-1fl/fl mice.Cpa3-Cre; Mcl-1fl/fl mice (n = 6) and Cpa3-Cre; Mcl-l+/+ control mice (n = 6) were sensitized by IP injection of 20 ng of anti-DNP IgE. Mice were challenged by IP injection of 1 mg DNP-HSA the next day. Body temperature measured at the indicated time points is shown as mean ± SD. **P < .05; **P < .01; and ***P < .001 versus corresponding values for Cpa3-Cre; Mcl-1+/+ mice at the indicated time points. P < .01 by 2-way ANOVA for the change in temperature in Cpa3-Cre; Mcl-lfl/fl mice versus Cpa3-Cre; Mcl-l+/+ mice. The data shown were pooled from the 2 independent experiments performed, each of which gave similar results. The death head symbols indicate when single mice in that group died.
IgE-dependent PSA is markedly reduced in Cpa3-Cre; Mcl-1fl/fl mice.Cpa3-Cre; Mcl-1fl/fl mice (n = 6) and Cpa3-Cre; Mcl-l+/+ control mice (n = 6) were sensitized by IP injection of 20 ng of anti-DNP IgE. Mice were challenged by IP injection of 1 mg DNP-HSA the next day. Body temperature measured at the indicated time points is shown as mean ± SD. **P < .05; **P < .01; and ***P < .001 versus corresponding values for Cpa3-Cre; Mcl-1+/+ mice at the indicated time points. P < .01 by 2-way ANOVA for the change in temperature in Cpa3-Cre; Mcl-lfl/fl mice versus Cpa3-Cre; Mcl-l+/+ mice. The data shown were pooled from the 2 independent experiments performed, each of which gave similar results. The death head symbols indicate when single mice in that group died.
Discussion
Our data support the conclusion that the hematopoietic survival factor Mcl-1 is required for normal levels of mouse mast cell and basophil development and/or survival in vitro or in vivo. These results are of particular interest given that whereas the majority (∼ 89%) of peritoneal mast cells in our Cpa3-Cre; Mcl-1fl/fl mice exhibited relatively high levels of Cpa3 promoter–driven expression of Cre recombinase, as assessed in Cpa3-Cre; mT/mG mice (Figure 2A), populations of each of 3 types of granulocytes (basophils, eosinophils, and neutrophils) in such mice exhibited high levels of Cpa3 promoter–driven expression of Cre recombinase in only a small proportion (∼ 13%-15%) of the cells (Figure 2D-F). However, we found that numbers of basophils were markedly reduced in Cpa3-Cre; Mcl-1fl/fl mice (Figure 4A), whereas numbers of blood, spleen, and BM eosinophils (Figure 4E and supplemental Figure 4A) and blood, BM, and peritoneal cavity neutrophils (Figure 4B and D) were not significantly affected (and there was a modest increase in the number of neutrophils in the spleen; Figure 4B). We do not know the reason for the differential effects of the Cpa3-Cre; Mcl-1fl/fl genotype on levels of basophils as opposed to eosinophils or neutrophils, but one possibility is that Mcl-1 is a more important antiapoptotic factor in basophils than in the other types of granulocytes.
In addition to exhibiting marked reductions in the numbers of mast cells and basophils, Cpa3-Cre; Mcl-1fl/fl mice exhibited significant reductions in the features of IgE-dependent biologic responses that are known to require mast cells or basophils. Therefore, both the tissue swelling (Figure 5A) and leukocyte infiltration (Figure 5C) associated with IgE- and mast cell–dependent PCA reactions23 were markedly and significantly reduced in Cpa3-Cre; Mcl-1fl/fl mice, with any minor tissue inflammation at the PCA reaction sites probably reflecting the presence of small residual populations of mast cells in the skin of some of the Cpa3-Cre; Mcl-1fl/fl mice (Figure 5D). The latter conclusion is supported by our observation that the tissue swelling and leukocyte infiltration associated with IgE-dependent PCA reactions in Cpa3-Cre; Mcl-1fl/fl mice were significantly increased (versus values in contralateral control ears) in mice that had been engrafted in the ear pinnae with 0.5 or 2.0 million in vitro–derived mast cells 8 weeks before eliciting the PCA reaction (supplemental Figure 6A-C and E-F). The latter result, and the demonstration that mast cells can be engrafted into the peritoneal cavity of Cpa3-Cre; Mcl-1fl/fl mice (supplemental Figure 6D), support the conclusion that Cpa3-Cre; Mcl-1fl/fl mice will be useful for studies of mast cell function in vivo. Cpa3-Cre; Mcl-1fl/fl mice also exhibited little or no hypothermia when tested for their ability to develop IgE-dependent PSA (Figure 7), as would be expected given that these mice have markedly reduced levels of the 2 key effector cells of such responses, mast cells and basophils.
In a form of IgE-dependent inflammation that has been shown to depend on basophils but not mast cells,25 both the tissue swelling (Figure 6A) and the leukocyte infiltration (Figure 6B) associated with the IgE-dependent CAI responses in the skin were essentially ablated in the Cpa3-Cre; Mcl-1fl/fl mice. Therefore, even though blood basophils are less severely depleted relative to wild-type numbers in Cpa3-Cre; Mcl-1fl/fl mice than are ear skin mast cells (with reductions of 74% versus 97%, respectively), the basophil deficiency was sufficient to result in a marked reduction in the IgE-dependent CAI response. It is possible that the number of basophils required to orchestrate IgE-dependent CAI exceeds that found in Cpa3-Cre; Mcl-1fl/fl mice. Alternatively, perhaps the residual blood basophils in Cpa3-Cre; Mcl-1fl/fl mice have impaired survival after recruitment to tissues and/or are functionally abnormal in ways that limit their ability to orchestrate IgE-dependent CAI.
It remains to be determined whether the induction of biologic responses other than the ones tested in the present study would reveal any significant abnormalities in hematopoietic lineages in addition to basophils and mast cells in Cpa3-Cre; Mcl-1fl/fl mice. We detected only minor abnormalities in lineages other than mast cells and basophils when we analyzed the mice under baseline conditions. Moreover, when we performed pilot experiments with a “mast cell–independent” model of antigen-induced chronic allergic inflammation of the airways,32-34 examination of C57BL/6J-Kit+/+ and C57BL/6J-KitW-sh/W-sh mice showed that the recruitment of leukocytes (including eosinophils and neutrophils, as assessed in bronchoalveolar lavage [BAL] fluid) and airway hyperreactivity to methacholine in this model can occur in C57BL/6J-KitW-sh/W-sh mice that virtually lack mast cells20,31,35,36 and that also have increased levels of neutrophils20,35,36 and basophils20 in the blood and BM (supplemental Figure 7). We found that Cpa3-Cre; Mcl-1fl/fl mice developed levels of airway hyperreactivity to methacholine that were similar to those of the corresponding Cpa3-Cre; Mcl-1+/+ control mice (supplemental Figure 7). However, compared with Cpa3-Cre; Mcl-1+/+ mice, Cpa3-Cre; Mcl-1fl/fl mice developed lower levels of BAL fluid eosinophils and higher levels of BAL fluid neutrophils. Additional work will be necessary to assess whether such differences (which did not achieve statistical significance) are confirmed in experiments with larger groups of mice, as well as to assess the extent to which any reproducible differences in levels of eosinophils, neutrophils, or other leukocytes in the BAL fluid in this model (or in any other mouse model of host defense or disease) reflected intrinsic abnormalities affecting these cells in Cpa3-Cre; Mcl-1fl/fl mice versus downstream consequences of their mast cell and/or basophil deficiency. Indeed, in both guinea pigs and mice, attempts to deplete basophils selectively also resulted in secondary reductions in eosinophils at sites of tick infestation37 or IgE-dependent CAI,38 respectively.
Whereas it will be important to continue to assess the phenotype of Cpa3-Cre; Mcl-1fl/fl mice, our findings to date—particularly the marked impairment in mast cell- and IgE-dependent PCA, IgE-dependent PSA, and basophil- and IgE-dependent CAI of the skin—suggest that Cpa3-Cre; Mcl-1fl/fl mice will be useful for in vivo analyses of the functions of mast cells and basophils in health and disease. With the notable exception of the small mast cell population in the spleen, Cpa3-Cre; Mcl-1fl/fl mice have a mast cell deficiency that is similar in magnitude to that observed in the genetically mast cell–deficient mice most commonly used for studies of mast cell function in vivo, WBB6F1-KitW/W-v mice and C57BL/6-KitW-sh/W-sh mice.31,39 However, unlike WBB6F1-KitW/W-v or C57BL/6-KitW-sh/W-sh mice, Cpa3-Cre; Mcl-1fl/fl mice lack mutations affecting c-Kit structure or expression, and therefore the phenotypic consequences of these mutations that affect populations other than mast cells will not have to be taken into account when interpreting experiments using Cpa3-Cre; Mcl-1fl/fl mice. Moreover, we9,10 and others11,12 have pointed out that mast cells and basophils may perform overlapping or complementary functions in several models of host defense or disease. Cpa3-Cre; Mcl-1fl/fl mice will permit us to examine such models in mice that are markedly and functionally deficient in both mast cells and basophils.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chen Liu, Christine Chang, Mariola Liebers-bach, and Justin Tang for excellent technical assistance, Dr Laurent Reber for critical advice regarding the manuscript, and Drs Hajime Karasuyama and Fu-Tong Liu for providing valuable reagents.
This study was supported by grants from the National Institutes of Health (AI070813, AI023990, and CA072074 to S.J.G.; HL087936 to C.-C.C.; and CA009151 to J.N.L.).
National Institutes of Health
Authorship
Contribution: J.N.L., C.-C.C., K.M., M.J.B., C.B.F., J.K., M.Y., and A.M.P. performed the experiments; J.N.L., C.-C.C., K.M., M.J.B., C.B.F., J.K., M.Y., M.T., A.M.P., and S.J.G. designed the research and analyzed the data; J.N.L. and S.J.G. wrote the manuscript; and all authors reviewed and helped to edit the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen J. Galli, MD, Department of Pathology, L-235, Stanford University School of Medicine, 300 Pasteur Dr, Stanford, CA 94305-5324; e-mail: sgalli@stanford.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal