Abstract
The regulation of cellular survival and apoptosis is of critical importance for the immune system to maintain immune homeostasis and to establish tolerance. Here, we demonstrate that the immune specific cell surface molecule Toso exhibits antiapoptotic effects on death receptor signaling by a novel regulatory mechanism involving the adaptor kinase RIP1. The antiapoptotic function of Toso depends on RIP1 ubiquitination and involves the recruitment of the death adaptor FADD to a Toso/RIP1 protein complex. In response to CD95L and TNFα, Toso promotes the activation of MAPK and NF-κB signaling pathways. Because of this relative augmentation of survival versus apoptotic signals, Toso raises the threshold for death receptor–mediated apoptosis. Our analysis of Toso-deficient mice revealed that Toso is essential for TNFα-mediated liver damage. Furthermore, the antiapoptotic function of Toso could be blocked by a Toso-specific monoclonal antibody, opening up new therapeutic prospects for the treatment of immune disorders and hematologic malignancies.
Introduction
One of the most well-characterized death receptors in the immune system is CD95 (Fas, APO-1). CD95 belongs to the TNF receptor superfamily. Expressed as preassociated homotrimers, CD95 on ligand binding directly conveys apoptotic signals. On ligand binding, CD95 assembles the death inducing signaling complex (DISC). The DISC consists of the death adaptor FADD (Fas associated death domain protein), procaspase-8, and procaspase-10.1,2 Efficient DISC formation provides a molecular scaffold concentrating cysteine proteases to induce autoproteolytic cleavage of caspase-8 and release of active caspase-8 that initiates the apoptotic program. Mutations of CD95 or its ligand have been found in autoimmune strains of mice and in patients with autoimmune lymphoproliferative syndrome (ALPS).3 These genetic abnormalities in CD95 result in the development of massive lymphoadenopathy and disruption of lymphocyte homeostasis because of increased survival of activated lymphocytes.4,5 Although CD95 has been mainly perceived as a death inducing receptor, recent findings paint a far more complex picture of CD95 and the DISC components by providing evidence that they can transmit both, death-inducing and cellular activation signals in response to the same ligand.6,7 Such nonapoptotic signaling activities of CD95 are thought to mediate proinflammatory responses in immune cells, neuronal tissue remodeling and promote tumor progression.7-9 The execution and regulation of CD95-mediated nonapoptotic signaling, in particular with respect to its contribution to tumorigenesis, is however still largely unclear.
Toso, also known as FAIM3, is a type I transmembrane protein belonging to the immunoglobulin gene superfamily. Toso was originally identified as a surface molecule with negative regulatory function on lymphocyte apoptosis.10 Recent studies have also implicated Toso in IgM binding.11 The expression of Toso is restricted to lymphoid organs, where it is particularly highly expressed in T, B and NK cells.12 Toso expression levels in T cells are regulated by cellular activation and it has been suggested that transient up-regulation of Toso in newly activated T cells might confer resistance to activation-induced apoptosis.10 In addition, recent studies have demonstrated a tight association of Toso overexpression with chronic lymphocytic leukemia (CLL),13,14 suggesting an involvement of Toso in neoplastic disorders. The biologic function of Toso is however still elusive and the antiapoptotic mechanism of Toso is only insufficiently understood.
Using miRNA-mediated specific Toso gene knock-down in human cells, as well as using Toso−/− mice, we here further characterize the antiapoptotic function of Toso. Toso associates with the adaptor kinase RIP1 and exhibits protective function on death receptor–mediated apoptosis by facilitating RIP1 ubiquitination. Moreover, our data demonstrate that in response to CD95L and TNFα-stimulation Toso promotes the activation of prosurvival signaling and in vivo is critically involved in TNFα-mediated tissue damage.
Methods
Cell Culture
BJAB, Jurkat and RIP1−/− Jurkat cells15 were cultured in RPMI 1640 supplemented with 10% FCS, penicillin/streptomycin (50 μg/mL of each) and 2 mM glutamine. Human peripheral blood T lymphocytes (PBT) were isolated by the Rosette Sep Kit (StemCell Technologies) and subsequent Ficoll-Hypaque density centrifugation of heparinized blood from healthy donors. Freshly isolated PBTs were activated via anti-CD3 (1 μg/mL, UCHT1, Pharmingen) and anti-CD28 (5 μg/mL, CD28.2, Pharmingen) mAbs and maintained in RPMI 1640 medium containing recombinant human IL-2 (25 U/mL, R&D Systems).
Apoptosis assay
Apoptosis was induced by treatment of cells with recombinant human CD95L (Apotech) for the times indicated in the figure legends. Alternatively, cells were also stimulated with 2 μg/mL anti-hCD95 (CH11) to induce apoptosis. For TNFα-induced apoptosis in murine T cells, T cells were isolated from spleen using a negative T cell selection kit (Miltenyi Biotech) and subsequently activated with 2 μg/mL anti-CD3 (2C11, BD) and 10 μg/mL of anti-CD28 (37.51, BD) for 24 hours. Apoptosis was then induced by stimulation of T cell blasts with 20 ng/mL TNFα (Apotech) for 24 hours. Apoptosis was analyzed by annexinV staining according to the manufacturer's instructions (BD Pharmingen). Apoptotic cells were quantified on a FACS Calibur flow cytometer and analyzed using FlowJo Version 8.8.6 software. In transient transfection experiments (Nucleofector), dead cells were removed using annexinV micro beads (Miltenyi Biotech) before apoptosis was induced.
Immunoprecipitation and Western blotting
Cells were stimulated for the indicated times with 200 ng/mL Flag-tagged CD95L (Apotech) or 2 μg/mL of anti-CD95 antibody (CH11) at 37°C and lysed with buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, phosphatase inhibitor cocktail (Sigma-Aldrich) and complete protease inhibitor cocktail (Boehringer). Immunoprecipitations were performed overnight at 4°C with anti-Flag M2-agarose beads (Sigma-Aldrich), ubiquitin or RIP1 antibody coupled protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology). Proteins from total cell lysates and immunprecipitates were resolved by SDS-PAGE and detected by Western blotting using anti-RIP1 (D94C12, Cell Signaling; BD Transduction Laboratories), anti-FLAG (M2, Sigma-Aldrich), anti-CD95 mAb (C20, Santa Cruz Biotechnology), anti-FADD (BD Transduction Laboratories), anti-ubiquitin (P4D1, Santa Cruz Biotechnology) and anti-actin (Sigma-Aldrich) antibodies.
Activation of Erk signaling pathways
To study Erk phosphorylation, cells were stimulated with 100 ng/mL CD95L for the indicated times. Cell lysates were analyzed by Western blotting using antibodies against phospho-Erk1/2 or total Erk1/2 (Cell Signaling Technology). Cells were also treated with 100 ng/mL PMA as a positive control. Alternatively, Erk phosphoylation was detected by flow cytometry. To this end, cells were fixed with 4% PFA and permeabilized with 0.2% TX-100 and then stained using a p-Erk specific antibody (Cell Signaling Technology).
TNFα-induced liver injury
Mice were sensitized by intraperitoneal administration of 10 mg D-galactosamine (Sigma-Aldrich). After 20 minutes 0.5 μg recombinant mouse TNF (R&D Systems) for ALT assay was injected intravenously. Liver damage was analyzed by alanine-aminotransferase (ALT) activity in the serum using a serum multiple biochemical analyzer (Ektachem DTSCII, Johnson & Johnson Inc).
Statistical analysis.
Data are expressed as mean ± SEM. Statistical significant differences between 2 different groups were analyzed using Student t test. Analysis including several groups were tested with Mantel Cox test. Statistically significant differences between treatment groups in experiments involving more than one analysis time point were calculated using 2-way ANOVA (repeated measurements). P values < .05 were considered as statistically significant. Statistical analysis was performed by 2-tailed Student t test (*P < .05, **P < .01). Error bars indicate SEM. For further materials and methods, see supplemental Methods (available on Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Toso is a novel negative regulator for CD95-induced apoptosis
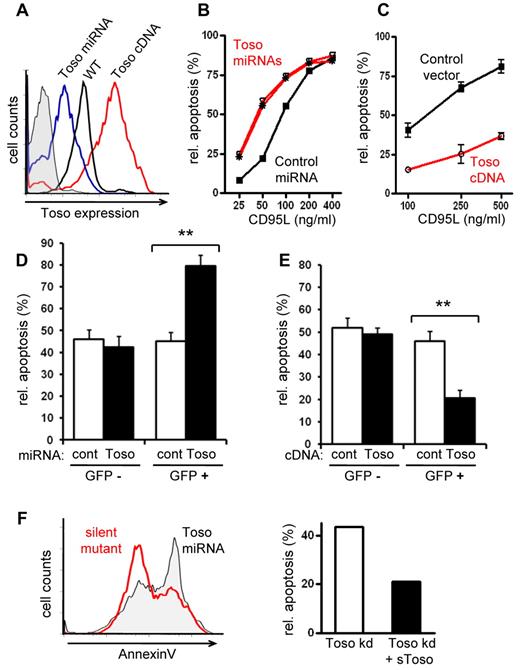
To investigate the biologic function of Toso we modulated Toso expression levels by using Toso specific gene knock-down and overexpression. Expression of miRNA specific for human Toso in the T and B lymphoma cell lines BJAB and Jurkat, as well as in primary human T lymphocytes, resulted in significantly reduced Toso expression compared with control miRNA expressing cells (Figure 1A and supplemental Figure 1A-C). On the other hand, increased Toso surface expression was achieved by overexpressing full-length human Toso cDNA (Figure 1A). To study the role of Toso in CD95-mediated apoptosis, cells were stimulated with CD95L and cellular apoptosis was quantified by annexin V staining. BJAB cells expressing Toso specific miRNA showed increased sensitivity to CD95L-induced apoptosis over time and over a wide range of CD95L concentrations, compared with control miRNA expressing cells (Figure 1B and supplemental Figure 1D). Thus, Toso knock-down causes a relative shift in the dose response curve to CD95L-induced apoptotis. Conversely, overexpression of Toso in BJAB cells had protective effects on CD95L-induced apoptosis (Figure 1C).
Toso is a negative regulator of CD95-induced apoptosis. (A) The modulation of Toso surface expression by Toso knock-down or overexpression. Representative flow cytometric analysis of BJAB cells stably expressing Toso specific miRNA (blue), control miRNA (black) or hToso cDNA (red). (B) Toso knock-down results in an increased sensitivity to CD95L-induced apoptosis. BJAB cells stably expressing control miRNA (black) or 2 different Toso specific miRNAs (red) were stimulated with various concentrations of CD95L for 5 hours. Apoptotic cells were assessed by staining with annexin V and relative apoptosis rates (%) were quantified. Representative data from > 10 independent experiments are shown. (C) Toso overexpression protects from CD95L-induced apoptosis. BJAB cells expressing either a control vector (black) or hToso cDNA (red) were stimulated for 5 hours with different concentrations of CD95L. Relative apoptosis was analyzed as in (B). Data shown are representative of more than 10 experiments. (D) CD95L-induced apoptosis in Toso knock-down primary T cells. Activated human peripheral blood T cells were lentivirally transduced with Toso specific miRNA/eGFP (black bars) or control miRNA/eGFP (white bars). Cells were stimulated with CD95L and apoptosis was assessed by staining with annexinV. Apoptotic cells were analyzed in GFP+ (transfected) and GFP− (nontransfected) cell populations. Data shown are representative of 3 experiments. Statistical analysis was performed by 2-tailed Student t test (*P < .05, **P < .01). (E) CD95L-induced apoptosis in Toso overexpressing primary T cells. Activated human peripheral blood T cells expressing a Toso-IRES-GFP construct (black bars) or control DNA (white bars). Apoptotic cells were analyzed by staining with annexinV in GFP+ (transfected) and GFP- (nontransfected) populations. Data shown are representative of 3 experiments. (F) Reconstitution of CD95-induced apoptosis in Toso knock-down cells. BJAB cells stably expressing Toso specific miRNAs were transiently transfected with a ‘miRNA resistant’ Toso cDNA containing silent mutations in the specific miRNA target site. Apoptosis was induced by CD95L stimulation for 5 hours. Flow cytometric histograms of mock treated control cells (gray filled) or Toso silent mutant expressing cells (red) are shown on the left. Relative percent of apoptosis is quantified on the right. Data shown are representative of 2 experiments.
Toso is a negative regulator of CD95-induced apoptosis. (A) The modulation of Toso surface expression by Toso knock-down or overexpression. Representative flow cytometric analysis of BJAB cells stably expressing Toso specific miRNA (blue), control miRNA (black) or hToso cDNA (red). (B) Toso knock-down results in an increased sensitivity to CD95L-induced apoptosis. BJAB cells stably expressing control miRNA (black) or 2 different Toso specific miRNAs (red) were stimulated with various concentrations of CD95L for 5 hours. Apoptotic cells were assessed by staining with annexin V and relative apoptosis rates (%) were quantified. Representative data from > 10 independent experiments are shown. (C) Toso overexpression protects from CD95L-induced apoptosis. BJAB cells expressing either a control vector (black) or hToso cDNA (red) were stimulated for 5 hours with different concentrations of CD95L. Relative apoptosis was analyzed as in (B). Data shown are representative of more than 10 experiments. (D) CD95L-induced apoptosis in Toso knock-down primary T cells. Activated human peripheral blood T cells were lentivirally transduced with Toso specific miRNA/eGFP (black bars) or control miRNA/eGFP (white bars). Cells were stimulated with CD95L and apoptosis was assessed by staining with annexinV. Apoptotic cells were analyzed in GFP+ (transfected) and GFP− (nontransfected) cell populations. Data shown are representative of 3 experiments. Statistical analysis was performed by 2-tailed Student t test (*P < .05, **P < .01). (E) CD95L-induced apoptosis in Toso overexpressing primary T cells. Activated human peripheral blood T cells expressing a Toso-IRES-GFP construct (black bars) or control DNA (white bars). Apoptotic cells were analyzed by staining with annexinV in GFP+ (transfected) and GFP- (nontransfected) populations. Data shown are representative of 3 experiments. (F) Reconstitution of CD95-induced apoptosis in Toso knock-down cells. BJAB cells stably expressing Toso specific miRNAs were transiently transfected with a ‘miRNA resistant’ Toso cDNA containing silent mutations in the specific miRNA target site. Apoptosis was induced by CD95L stimulation for 5 hours. Flow cytometric histograms of mock treated control cells (gray filled) or Toso silent mutant expressing cells (red) are shown on the left. Relative percent of apoptosis is quantified on the right. Data shown are representative of 2 experiments.
To extend our studies beyond BJAB cells, we next analyzed the role of Toso in CD95-mediated apoptosis with primary human T lymphocytes (PBTs). PBTs were activated via CD3/CD28 and then transfected with cocistronic lentiviral constructs expressing miRNA for Toso or control miRNA together with GFP. GFP+ T cells expressing Toso specific miRNA exhibited significantly higher rates of CD95-induced apoptosis compared with nontransfected (GFP−) cells or cells transfected with control miRNA (Figure 1D). Furthermore, in overexpression experiments we transfected activated PBTs with full-length human Toso cDNA followed by an IRES-GFP cassette to monitor expression. PBTs overexpressing Toso were less sensitive to CD95-induced apoptosis compared with control transfected cells or nontransfected (GFP−) cells (Figure 1E).
The specificity of Toso specific miRNAs was confirmed in experiments in which the increased sensitivity to CD95-induced apoptosis in Toso knock-down cells could be reversed by reintroducing a miRNA resistant Toso construct containing silent mutations in the miRNA target site (Figure 1F). Together these data clearly demonstrate that CD95-mediated apoptosis is negatively regulated by the level of Toso gene expression.
Furthermore, in clear contrast to previous reports,11 in our experimental system we did not detect any specific binding of IgM to Toso (supplemental Figures 11-12). Together with the observation that the protective effect of Toso was seen in response to stimulation with the physiologic CD95 ligand (Figure 1), this argues against the proposed involvement of IgM in the antiapoptotic function of Toso.11 Noteworthy, etoposide-induced apoptosis, which acts via a death receptor independent intrinsic apoptotic pathway, was unaffected by Toso gene knock-down or overexpression (data not shown). Thus, the inhibitory function of Toso on cellular apoptosis appears to act specifically on death receptor–mediated apoptotic signaling pathways.
The antiapoptotic function of Toso in CD95 signaling was not only observed in type I BJAB cells and in primary T cells, in which death receptor–induced apoptosis depends on DISC formation and caspase-8 activation, but also in type II Jurkat cells, which are more dependent on a mitochondrial amplification loop and exhibit only poor and delayed DISC formation and caspase-8 activation. Similar to the effects observed in type I cells, specific knock-down of Toso in type II Jurkat cells rendered the cells more susceptible to CD95-mediated apoptosis, whileoverexpression of Toso had protective effects (supplemental Figure 4A).
In Jurkat cells, triggering of death receptors in the presence of caspase inhibitors induces necrotic cell death.16-18 Interestingly, the protective function of Toso was also observed in TNF-R and TRAIL-R induced nonapoptotic, caspase-independent cell death (supplemental Figure 4B). Together with the antiapoptotic effects of Toso in type I and type II cells, these data suggest that Toso generally exhibits a protective function on death receptor–mediated forms of cell death. In addition, unlike earlier reports on type II Jurkat cells,10,19 we observed no significant increase in caspase-8 cleavage in BJAB (type I) Toso knock-down cells, although these cells exhibit enhanced apoptosis on CD95 stimulation compared with control cells (supplemental Figure 4C). Together, our results indicate that Toso exhibits its antiapoptotic function by acting on signaling events that either lie downstream or in parallel to the activation of initiator caspase-8.
RIP1 ubiquitination is required for the antiapoptotic function of Toso
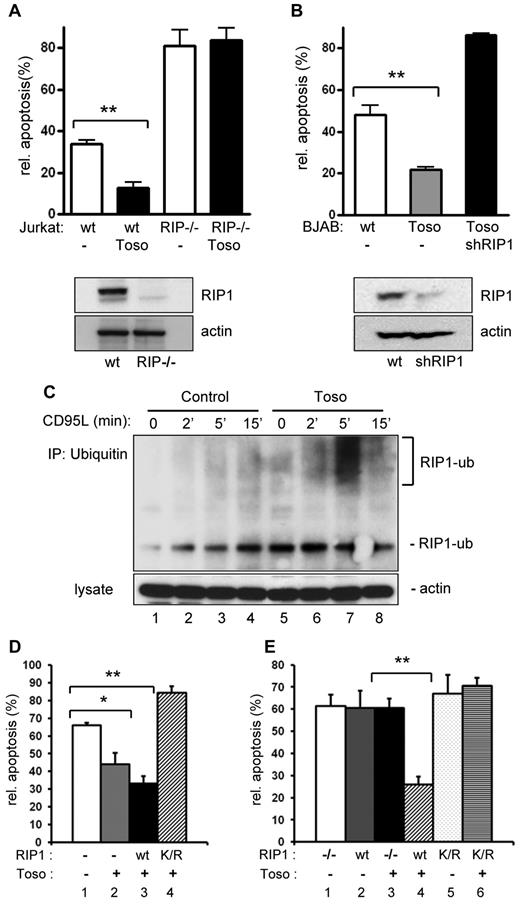
As an intriguing possibility we next explored whether Toso acts by promoting death receptor–mediated cellular survival pathways. A key molecule in the activation of survival signaling pathways on triggering of TNF-receptor family members is the adaptor kinase RIP1 (receptor interacting protein 1).15,20,21 Therefore, to further define the molecular mechanisms underlying the antiapoptotic effects of Toso, we investigated the potential involvement of RIP1 in Toso function. While overexpression of Toso clearly exhibits antiapoptotic effects in normal Jurkat cells, Toso overexpression had no protective effects on CD95-induced apoptosis in RIP1 deficient Jurkat cells (Figure 2A). Using shRNA-mediated gene knock-down, we also efficiently ablated RIP1 expression in BJAB cells stably expressing Toso (Figure 2B, lower panel). Similar to the results on RIP1 deficient Jurkat cells, apoptosis rates were significantly higher in Toso overexpressing and RIP depleted BJAB cells, compared with Toso overexpressing control cells (Figure 2B).
RIP1 is required for the antiapoptotic function of Toso and Toso facilitates RIP1 ubiquitination in response to CD95 stimulation. (A) Wild-type or RIP1 deficient Jurkat cells were transfected with a control or a Toso construct. Cells were stimulated with CD95L for 5 hours and apoptosis was assessed by annexin V staining. RIP1 (top) and actin (bottom) expression levels in cell lysates are shown by Western blotting. Data are representative of 3 experiments. Statistical analysis: Student t test (*P < .05, **P < .01). (B) BJAB cells stably expressing Toso were transfected with 3 different RIP1 specific shRNA constructs or mock treated. CD95-induced apoptosis was analyzed as in panel A. The knock-down efficiency of RIP1 was monitored by immunoblotting for RIP1 (top). Actin was used as a control for protein loading (bottom). Data are representative of 2 experiments. (C) Jurkat cells were transiently transfected with a control or a Toso construct. Cells were stimulated with CD95L for the indicated times and ubiquitinated proteins were isolated by immunoprecipitation (IP) using an antiubiquitin antibody. Ubiquitinated endogenous RIP1 was detected in immunoprescipitates using RIP1 antibody (top) and total lysates were probed for actin (bottom). Data are representative of 2 experiments. (D) The antiapoptotic function of Toso depends on RIP1 ubiquitination at lysine 377. Wild-type Jurkat cells were transiently transfected with control pIRES-GFP vector (lane 1), Toso-IRES-GFP only (lane 2), wt RIP1-FLAG and Toso-IRES-GFP (lane 3), or RIP1-K377R-FLAG (K/R) and Toso-IRES-GFP (lane 4). Cells were stimulated with CD95L for 5 hours. Apoptotic cells in the GFP+ population were determined by annexin V staining. Representative data from 5 independent experiments are shown. Statistical analysis: Student t test (*P < .05, **P < .01). (E) RIP1 deficient Jurkat (RIP1−/−) cells were transiently transfected with control pIRES-GFP vector (lane 1), wt RIP1-FLAG and control pIRES-GFP vector (lane 2), Toso-IRES-GFP only (lane 3), wt RIP1-FLAG and Toso-IRES-GFP (lane 4), RIP1-K377R-FLAG (K/R) and control pIRES-GFP vector (lane 5), or RIP1-K377R-FLAG (K/R) and Toso-IRES-GFP (lane 6). Cells were stimulated with CD95L for 5 hours. Apoptotic cells in the GFP+ population were determined by annexin V staining. Representative data from 3 independent experiments are shown.
RIP1 is required for the antiapoptotic function of Toso and Toso facilitates RIP1 ubiquitination in response to CD95 stimulation. (A) Wild-type or RIP1 deficient Jurkat cells were transfected with a control or a Toso construct. Cells were stimulated with CD95L for 5 hours and apoptosis was assessed by annexin V staining. RIP1 (top) and actin (bottom) expression levels in cell lysates are shown by Western blotting. Data are representative of 3 experiments. Statistical analysis: Student t test (*P < .05, **P < .01). (B) BJAB cells stably expressing Toso were transfected with 3 different RIP1 specific shRNA constructs or mock treated. CD95-induced apoptosis was analyzed as in panel A. The knock-down efficiency of RIP1 was monitored by immunoblotting for RIP1 (top). Actin was used as a control for protein loading (bottom). Data are representative of 2 experiments. (C) Jurkat cells were transiently transfected with a control or a Toso construct. Cells were stimulated with CD95L for the indicated times and ubiquitinated proteins were isolated by immunoprecipitation (IP) using an antiubiquitin antibody. Ubiquitinated endogenous RIP1 was detected in immunoprescipitates using RIP1 antibody (top) and total lysates were probed for actin (bottom). Data are representative of 2 experiments. (D) The antiapoptotic function of Toso depends on RIP1 ubiquitination at lysine 377. Wild-type Jurkat cells were transiently transfected with control pIRES-GFP vector (lane 1), Toso-IRES-GFP only (lane 2), wt RIP1-FLAG and Toso-IRES-GFP (lane 3), or RIP1-K377R-FLAG (K/R) and Toso-IRES-GFP (lane 4). Cells were stimulated with CD95L for 5 hours. Apoptotic cells in the GFP+ population were determined by annexin V staining. Representative data from 5 independent experiments are shown. Statistical analysis: Student t test (*P < .05, **P < .01). (E) RIP1 deficient Jurkat (RIP1−/−) cells were transiently transfected with control pIRES-GFP vector (lane 1), wt RIP1-FLAG and control pIRES-GFP vector (lane 2), Toso-IRES-GFP only (lane 3), wt RIP1-FLAG and Toso-IRES-GFP (lane 4), RIP1-K377R-FLAG (K/R) and control pIRES-GFP vector (lane 5), or RIP1-K377R-FLAG (K/R) and Toso-IRES-GFP (lane 6). Cells were stimulated with CD95L for 5 hours. Apoptotic cells in the GFP+ population were determined by annexin V staining. Representative data from 3 independent experiments are shown.
As ubiquitination of RIP1 has been reported to be critical for the activation of NF-κB signaling pathways by TNFα22-26 and RIP1 is also involved in the function of Toso, we hypothesized that Toso might regulate RIP1 ubiquitination. Jurkat T cells were stimulated with CD95L for various times and ubiquitinated proteins were isolated by immunoprecipitation using ubiquitin-specific mAbs. Subsequent immunoblotting for RIP1 indicated that CD95-triggering strongly induces poly-ubiquitination of RIP1 (Figure 2C). The formation of poly-ubiquitinated (high molecular weight) RIP1 was detected within 5 minutes after CD95 stimulation in Toso overexpressing cells, but was poorly detectable in control cells. In addition, in contrast to control cells, high levels of mono-ubiquitinated RIP1 were constitutively detected in Toso overexpressing cells. Reciprocal immunoprecipitation using anti-RIP1 antibody followed by antiubiquitin staining similarly detected increased RIP1 ubiquitination in Toso overexpressing cells (supplemental Figure 5B). In Toso depleted cells CD95-induced RIP1 ubiquitination was completely abolished, as RIP1 could not be detected in ubiquitin immunoprecipitates of CD95-stimulated Toso miRNA expressing cells (supplemental Figure 5A). Together, our data demonstrate that Toso expression levels control the ubiquitination of RIP1 during CD95-mediated signal transduction.
Site-specific ubiquitination of lysine 377 (K377) of RIP1 has recently been demonstrated to mediate efficient activation of the IKK complex by TNFα. To examine the potential involvement of K377 of RIP1 in the antiapoptotic function of Toso, we transfected Jurkat cells with Toso-IRES-GFP alone or in combination with either wild-type (wt) RIP1, or the RIP1-K377R mutant, that does not undergo ligand-dependent ubiquitination and fails to activate IKK on TNFα stimulation.23 Cells were then stimulated with CD95L and apoptotic cell death was assessed (Figure 2D). As seen before, reduced apoptosis was detected in Toso overexpressing cells compared with control cells. Overexpression of both, Toso and RIP1-wt conferred only minimal additional protection from CD95-mediated apoptosis. However, Jurkat cells overexpressing Toso together with RIP1-K377R were significantly more susceptible to CD95-induced apoptosis than cells overexpressing Toso alone or in combination with RIP1-wt (Figure 2D). Thus, Toso could not efficiently protect from CD95-mediated apoptosis in RIP1-K377R expressing cells. These results were further substantiated by experiments in RIP1 deficient Jurkat cells. As shown before, Toso overexpression does not protect from CD95-induced apoptosis in the absence of RIP1 (Figure 2A-E). In addition, re-expression of wt RIP1 alone had no influence on apoptosis. Only when RIP−/− Jurkat cells were transiently transfected with Toso and wt RIP1 expression constructs, CD95-induced apoptosis was significantly reduced (Figure 2E). Importantly, Toso could not protect from CD95-mediated apoptosis in the presence of RIP1-K377R.
Together, our data suggest that Toso promotes the ubiquitination of RIP1 at K377 in response to CD95-triggering, and this ubiquitination step is an essential requirement for the antiapoptotic function of Toso.
Toso associates with FADD in a RIP1-dependent fashion
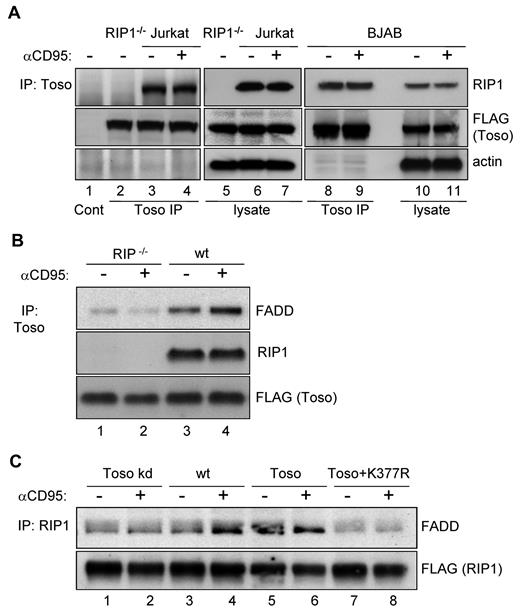
We next examined whether RIP1 and Toso would either constitutively or inducibly associate with each other. We immunoprecipitated Toso from cellular lysates of CD95-stimulated or unstimulated control cells. Independently of cellular stimulation, RIP1 specifically coimmunoprecipitated with Toso in Jurkat T cells (Figure 3A lanes 3-4). To control for the specificity of the RIP1 detection, we included RIP1 deficient Jurkat cells in our analysis (Figure 3A lane 2). RIP1 was also found in Toso immunoprecipitates from CD95-stimulated and unstimulated BJAB cells (Figure 3A lanes 8-9).
Toso forms a trimolecular complex with RIP1 and FADD. (A) Toso constitutively associates with RIP1. Wild-type (lanes 1, 3, 4, 6, 7), RIP deficient (lanes 2,5) Jurkat cells or wt BJAB cells (lanes 8-11) were transiently transfected with a Toso-FLAG construct. Cell lysates from anti-hCD95 (CH11) treated and untreated cells were immunoprecipitated (IP) using anti-FLAG coupled agarose. The control IP of wt Jurkat cells using mouse Ig coupled agarose is shown in lane 1. Control or Toso immunoprecipitates and corresponding total cell lysates were analyzed by Western blotting for RIP1, FLAG and actin. Representative data from 3 independent experiments are shown. (B) RIP1 is required for the association of Toso with FADD. RIP deficient Jurkat cells (lanes 1-2) or wt Jurkat cells (lanes 3-4) were transfected with Toso-FLAG and stimulated with anti-CD95 (CH11) for 15 minutes or left untreated. Cell lysates were immunoprecipitated with anti-FLAG coupled agarose. Immunoprecipitates were analyzed by Western blotting for RIP1, FLAG or FADD. Data are representative of 3 experiments. (C) Toso and RIP1 ubiquitination promote the interaction of RIP1 with FADD. Jurkat cells stably expressing Toso miRNA (lanes 1-2), wt Jurkat (lanes 3-4), or Toso overexpressing Jurkat cells (lanes 5-8) were transfected with FLAG-RIP1 (lanes 1-6) or FLAG-K377R RIP1 (lanes 7-8). Untreated cells or cells stimulated with anti-CD95 (CH11) for 15 minutes were immunoprecipitated with anti-FLAG coupled agarose. Proteins bound to the RIP1 complexes were immunoblotted with antibodies against FADD (top) or FLAG (bottom). Data are representative of 2 experiments.
Toso forms a trimolecular complex with RIP1 and FADD. (A) Toso constitutively associates with RIP1. Wild-type (lanes 1, 3, 4, 6, 7), RIP deficient (lanes 2,5) Jurkat cells or wt BJAB cells (lanes 8-11) were transiently transfected with a Toso-FLAG construct. Cell lysates from anti-hCD95 (CH11) treated and untreated cells were immunoprecipitated (IP) using anti-FLAG coupled agarose. The control IP of wt Jurkat cells using mouse Ig coupled agarose is shown in lane 1. Control or Toso immunoprecipitates and corresponding total cell lysates were analyzed by Western blotting for RIP1, FLAG and actin. Representative data from 3 independent experiments are shown. (B) RIP1 is required for the association of Toso with FADD. RIP deficient Jurkat cells (lanes 1-2) or wt Jurkat cells (lanes 3-4) were transfected with Toso-FLAG and stimulated with anti-CD95 (CH11) for 15 minutes or left untreated. Cell lysates were immunoprecipitated with anti-FLAG coupled agarose. Immunoprecipitates were analyzed by Western blotting for RIP1, FLAG or FADD. Data are representative of 3 experiments. (C) Toso and RIP1 ubiquitination promote the interaction of RIP1 with FADD. Jurkat cells stably expressing Toso miRNA (lanes 1-2), wt Jurkat (lanes 3-4), or Toso overexpressing Jurkat cells (lanes 5-8) were transfected with FLAG-RIP1 (lanes 1-6) or FLAG-K377R RIP1 (lanes 7-8). Untreated cells or cells stimulated with anti-CD95 (CH11) for 15 minutes were immunoprecipitated with anti-FLAG coupled agarose. Proteins bound to the RIP1 complexes were immunoblotted with antibodies against FADD (top) or FLAG (bottom). Data are representative of 2 experiments.
Together, these data indicate that Toso constitutively associates with RIP1. Moreover, the inhibitory activity of Toso is largely abolished in RIP1 knock-down or null mutant cells, suggesting that Toso uses RIP1 as a downstream effector molecule to mediate its antiapoptotic function.
The C-terminus of mouse Toso has been described to bind to FADD in in vitro binding assays.19 To investigate whether an association between Toso and FADD also exists in vivo and whether this association may be influenced by CD95-stimulation, we immunoprecipitated Toso from lysates of CD95-stimulated and unstimulated Jurkat cells. As seen before (Figure 3A), Toso was found to be constitutively associated with RIP1 (Figure 3B lanes 3-4). Moreover, FADD could be clearly detected in Toso immunoprecipitates from lysates of nonstimulated wt Jurkat cells, and this association was significantly enhanced on CD95-stimulation (Figure 3B lanes 3-4). Importantly, the association of Toso with FADD was almost completely abolished in RIP1 deficient Jurkat cells, independently of CD95-stimulation (Figure 3B lanes 1-2). Thus, in vivo the association of Toso with FADD surprisingly is dependent on RIP1.
The molecular interactions between RIP1, Toso and FADD were further examined in immunoprecipitation experiments, where we expressed Flag-tagged RIP1 either in control, Toso knock-down or Toso overexpressing Jurkat cells. After stimulation with anti-CD95 for 0 or 15 minutes, cells were lysed and subjected to immunoprecipitation. As shown in Figure 3C (lanes 3,4), low levels of RIP1 constitutively associate with FADD in nonstimulated Jurkat cells and this association significantly increases on CD95 stimulation. Compared with normal Jurkat cells, the constitutive association of FADD with RIP1 was substantially higher in Toso overexpressing cells, where CD95-triggering however did not further enhance the association (Figure 3C lanes 5,6). Conversely, only low amounts of FADD were detected in RIP1 immunoprecipitates from Toso knock-down cells and in these cells we also observed no further recruitment of FADD to RIP1 in response CD95-stimulation (Figure 3C lanes 1,2). Hence, the interaction between RIP1 and FADD is significantly influenced by the expression of Toso. Interestingly, we detected only a very weak association of FADD with the RIP1-K377R ubiquitination mutant in unstimulated Toso overexpressing cells and no further induction of FADD binding to RIP1-K377R could be observed on CD95 stimulation (Figure 3C lanes 7-8).
Together, the data suggest that in living cells Toso, RIP1 and FADD form a trimolecular protein complex. Deleting either Toso or RIP1 disrupts this protein complex, while CD95 signaling seems to enhance complex formation. Furthermore, the weak association of FADD with the RIP1-K377R mutant suggests, that RIP1 ubiquitination favors complex formation.
Toso promotes the activation of prosurvival signals
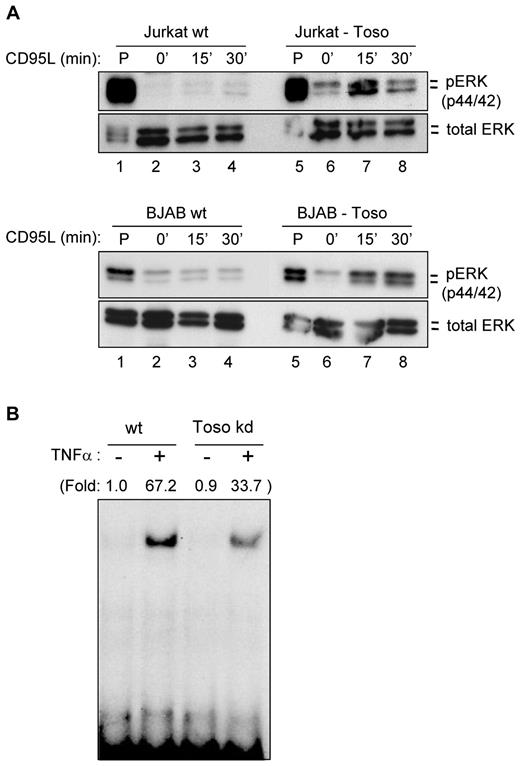
RIP1 is involved in TNFα-induced NF-κB signaling and MAP kinase activation.15,20,21,25,28 As RIP1 is also mediating the antiapoptotic effects of Toso, we examined whether in response to CD95-stimulation Toso might induce the activation of prosurvival signals, such as NF-κB and/or MAP kinase signaling pathways. Wild-type Jurkat cells stimulated with CD95L showed no induction of Erk1/2 phosphorylation (Figure 4A top panel lanes 2-4). Surprisingly, in Toso overexpressing Jurkat cells CD95L stimulation readily induced the activation of Erk1/2 (Figure 4A top panel lanes 6-8). Similar results were obtained with BJAB cells: While CD95-ligation did not induce any measurable Erk1/2 activation, Toso overexpressing BJAB cells show CD95-induced phosphorylation of Erk1/2 (Figure 4A bottom panel). Thus, these data demonstrate that survival signaling pathways can be activated on CD95-triggering in cells in which Toso expression is up-regulated.
Toso activates survival signaling pathways. (A) Toso activates Erk signaling pathways in response to CD95 stimulation. Jurkat cells (top panel) or BJAB cells (bottom panel) stably expressing Toso or respective wt control cells were stimulated with 100 ng/mL CD95L for the indicated times. Cell lysates were analyzed for Erk1/2 phosphorylation (top) or total Erk1/2 (bottom) expression. Cells were also treated with PMA as a positive control. Data are representative of 3 experiments. Statistical analysis: Student t test (**P < .01). (B) Toso activates NF-κB in response to TNFα stimulation. Toso knock-down Jurkat cells or wt Jurkat cells were stimulated with 10 ng/mL TNFα for 20 minutes. NF-κB binding activity was analyzed by electrophoretic mobility shift assay. Data are representative of 2 experiments.
Toso activates survival signaling pathways. (A) Toso activates Erk signaling pathways in response to CD95 stimulation. Jurkat cells (top panel) or BJAB cells (bottom panel) stably expressing Toso or respective wt control cells were stimulated with 100 ng/mL CD95L for the indicated times. Cell lysates were analyzed for Erk1/2 phosphorylation (top) or total Erk1/2 (bottom) expression. Cells were also treated with PMA as a positive control. Data are representative of 3 experiments. Statistical analysis: Student t test (**P < .01). (B) Toso activates NF-κB in response to TNFα stimulation. Toso knock-down Jurkat cells or wt Jurkat cells were stimulated with 10 ng/mL TNFα for 20 minutes. NF-κB binding activity was analyzed by electrophoretic mobility shift assay. Data are representative of 2 experiments.
As Toso also acts on TNFα-mediated signaling pathways, we next examined the effects of Toso on TNFα-mediated NF-κB activation. Wild-type or Toso knock-down Jurkat T cells were stimulated with TNFα and NF-κB activation was determined by electrophoretic mobility shift assay (EMSA). These experiments revealed that TNFα-induced NF-κB binding activity was significantly lower in Toso knock-down Jurkat cells compared with wt Jurkat cells (Figure 4B). Conversely, Toso overexpression resulted in increased TNFα-mediated NF-κB activation (supplemental Figure 7). In an additional approach we attempted to interfere with the antiapoptotic function of Toso, as well as with the activation of cell survival pathways using Toso-specific antibodies. By genetic immunization we have developed monoclonal antibodies (mAb A36 and A38) that specifically recognize the extracellular portion of human Toso (supplemental Figure 8). Anti-Toso mAb A36 had no effect on CD95-mediated apoptosis of human primary T lymphocytes; however, anti-Toso mAb A38 significantly enhanced CD95-induced apoptosis compared with control-Ig treated cells (Figure 5A). Importantly, the addition of anti-Toso mAb A38 specifically increased CD95-mediated apoptosis, but did not induce apoptosis/cell death by its own. We next investigated whether anti-Toso mAbs could also modulate cell survival signaling pathways, such as MAPK-pathways. Human primary T lymphocytes were stimulated with TNFα and analyzed for Erk-activation. Addition of anti-Toso mAb A36 did not influence TNFα-induced Erk-phosphorylation compared with control-IgG treatment (Figure 5B). However, preincubation with anti–Toso mAb A38 almost completely blocked TNFα-mediated Erk-activation in human T cells (Figure 5B). Thus, anti-Toso mAb A38, but not mAb A36, specifically blocks the antiapoptotic function of Toso, as well as the TNFα-mediated activation of MAPK signaling pathways.
Toso specific monoclonal antibody A38 blocks survival signaling pathways. (A) Anti–human Toso mAb A38 enhances CD95-mediated apoptosis. Human primary T cells were preincubated for 60 minutes with 2 μg/mL of anti–human Toso mAb A36, A38 or control rat IgG. Cells were then stimulated for 16 hours with 100 ng/mL CD95L and apoptosis was assessed by annexin V staining. Representative flow cytometric histograms from cells treated with control IgG (gray filled), anti–human Toso mAb A36 (blue) or anti–human Toso mAb A38 (red) are shown on the left. Relative % of apoptosis is quantified on the right. Data are representative of 4 experiments. Statistical analysis: Student t test (**P < .01). (B) Anti–human Toso mAb A38 inhibits TNFα-mediated Erk-phosphorylation. Human primary T cells were preincubated for 60 minutes with 2 μg/mL human Toso mAb A36, A38 or control rat IgG. Cells were then stimulated for 10 minutes with 100 ng/mL TNFα. Erk-phosphorylation was examined by intracellular flow cytometric analysis using a phospho-Erk specific antibody. Representative flow cytometric histograms from cells treated with control IgG (gray filled), anti–human Toso mAb A36 (blue) or anti–human Toso mAb A38 (red) are shown on the left. Relative % of Erk-phosphorylation is quantified on the right. Data are representative of 3 experiments. Statistical analysis: Student t test (**P < .05).
Toso specific monoclonal antibody A38 blocks survival signaling pathways. (A) Anti–human Toso mAb A38 enhances CD95-mediated apoptosis. Human primary T cells were preincubated for 60 minutes with 2 μg/mL of anti–human Toso mAb A36, A38 or control rat IgG. Cells were then stimulated for 16 hours with 100 ng/mL CD95L and apoptosis was assessed by annexin V staining. Representative flow cytometric histograms from cells treated with control IgG (gray filled), anti–human Toso mAb A36 (blue) or anti–human Toso mAb A38 (red) are shown on the left. Relative % of apoptosis is quantified on the right. Data are representative of 4 experiments. Statistical analysis: Student t test (**P < .01). (B) Anti–human Toso mAb A38 inhibits TNFα-mediated Erk-phosphorylation. Human primary T cells were preincubated for 60 minutes with 2 μg/mL human Toso mAb A36, A38 or control rat IgG. Cells were then stimulated for 10 minutes with 100 ng/mL TNFα. Erk-phosphorylation was examined by intracellular flow cytometric analysis using a phospho-Erk specific antibody. Representative flow cytometric histograms from cells treated with control IgG (gray filled), anti–human Toso mAb A36 (blue) or anti–human Toso mAb A38 (red) are shown on the left. Relative % of Erk-phosphorylation is quantified on the right. Data are representative of 3 experiments. Statistical analysis: Student t test (**P < .05).
Toso deficiency protects from TNFα-induced liver damage
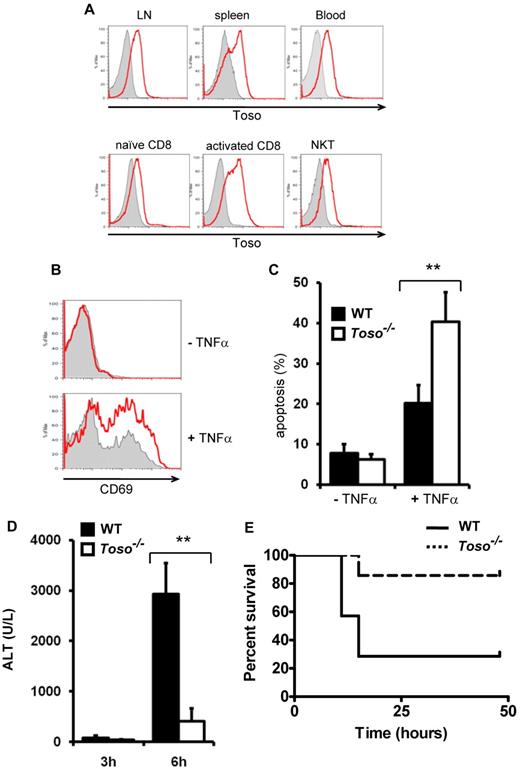
To investigate the physiologic function of Toso, Toso deficient mice were generated (supplemental Figure 9). Toso−/− mice were fertile, appeared healthy and did not show any gross physical abnormalities. T cell development in the thymus was normal, and Toso−/− mice also had normal numbers of T cells in spleen, lymph nodes and peripheral blood (supplemental Figure 10). B cell numbers, however, were slightly reduced in peripheral lymphoid organs. In mice, Toso expression was detected on the surface of leukocytes in spleen, lymph node and blood (Figure 6A), whereas no expression of Toso was found on thymocytes (data not shown). On resting peripheral CD8+ T cells Toso was expressed at low levels and its expression was dramatically increased on T cell activation (Figure 6A). Notably, Toso is constitutively expressed on NKT cells. Flow cytometric analysis confirmed that Toso-null mice had no detectable Toso protein expression (Figure 6A).
Toso deficiency results in defective TNFα signaling and protects from TNFα-mediated liver damage. (A) Toso expression in peripheral lymphoid organs. Leukocytes from lymph node (LN), spleen, peripheral blood, naive CD8+ T cells, CD3/CD28 activated CD8+ T cells and NKT cells (DX5+CD3+) were stained with anti–mouse Toso antibody. Flow cytometric histograms of Toso−/− cells (gray filled) and Toso+/+ cells (red) are shown in overlays. Data are representative of 2 experiments using 4 mice per group. (B) Toso deficiency results in decreased TNFα-induced coactivation of CD8+ T cells. Purified naive CD8+ T cells from spleen of Toso+/+ or Toso−/− mice were incubated with 2 ng/mL of IL-2 (top panel) or IL-2 + 20 ng/mL of TNFα (bottom panel) for 12 hours and analyzed for CD69 surface expression. Flow cytometric histograms of Toso−/− cells (gray filled) and Toso+/+ cells (red) are shown in overlays. Data shown are representative of 3 experiments using 6 mice per group. (C) Toso deficiency results in increased TNFα-induced apoptosis in CD8+ T cells. CD8+ T cells were stimulated for 24 hours via CD3/CD28. Subsequently, activated CD8+ T blasts were stimulated with 20 ng/mL of TNFα for another 24 hours or left unstimulated. Cellular apoptosis of Toso+/+ (black bars) or Toso−/− (white bars) CD8+ T cells were assessed by staining with annexinV and 7-AAD. Results shown are mean percentages ± SEM of apoptotic cells (n = 6/group). Data shown are representative of 3 experiments. Statistical analysis: Student t test (**P < .01). (D) Toso deficiency protects from TNFα-induced liver injury. Toso+/+ and Toso−/− mice were injected intraperitoneally with GalN followed an IV administration of TNFα. Liver damage was analyzed by measuring alanine-aminotransferase (ALT) activity in the serum of Toso+/+ (black) and Toso−/− (white) mice at 4 and 6 hours after TNFα administration. Results shown are mean values ± SEM (n = 5/group). Statistical analysis: Student t test (**P < .01). Data shown are representative of 3 experiments. (E) Survival of wt C57BL/6 and Toso-deficient mice. Toso+/+ and Toso−/− mice were injected intraperitoneally with GalN followed an intravenous administration of TNFα. Results shown are from 7 mice per genotype (n = 7, P = .027, Mantel Cox test). Data shown are representative of 3 experiments.
Toso deficiency results in defective TNFα signaling and protects from TNFα-mediated liver damage. (A) Toso expression in peripheral lymphoid organs. Leukocytes from lymph node (LN), spleen, peripheral blood, naive CD8+ T cells, CD3/CD28 activated CD8+ T cells and NKT cells (DX5+CD3+) were stained with anti–mouse Toso antibody. Flow cytometric histograms of Toso−/− cells (gray filled) and Toso+/+ cells (red) are shown in overlays. Data are representative of 2 experiments using 4 mice per group. (B) Toso deficiency results in decreased TNFα-induced coactivation of CD8+ T cells. Purified naive CD8+ T cells from spleen of Toso+/+ or Toso−/− mice were incubated with 2 ng/mL of IL-2 (top panel) or IL-2 + 20 ng/mL of TNFα (bottom panel) for 12 hours and analyzed for CD69 surface expression. Flow cytometric histograms of Toso−/− cells (gray filled) and Toso+/+ cells (red) are shown in overlays. Data shown are representative of 3 experiments using 6 mice per group. (C) Toso deficiency results in increased TNFα-induced apoptosis in CD8+ T cells. CD8+ T cells were stimulated for 24 hours via CD3/CD28. Subsequently, activated CD8+ T blasts were stimulated with 20 ng/mL of TNFα for another 24 hours or left unstimulated. Cellular apoptosis of Toso+/+ (black bars) or Toso−/− (white bars) CD8+ T cells were assessed by staining with annexinV and 7-AAD. Results shown are mean percentages ± SEM of apoptotic cells (n = 6/group). Data shown are representative of 3 experiments. Statistical analysis: Student t test (**P < .01). (D) Toso deficiency protects from TNFα-induced liver injury. Toso+/+ and Toso−/− mice were injected intraperitoneally with GalN followed an IV administration of TNFα. Liver damage was analyzed by measuring alanine-aminotransferase (ALT) activity in the serum of Toso+/+ (black) and Toso−/− (white) mice at 4 and 6 hours after TNFα administration. Results shown are mean values ± SEM (n = 5/group). Statistical analysis: Student t test (**P < .01). Data shown are representative of 3 experiments. (E) Survival of wt C57BL/6 and Toso-deficient mice. Toso+/+ and Toso−/− mice were injected intraperitoneally with GalN followed an intravenous administration of TNFα. Results shown are from 7 mice per genotype (n = 7, P = .027, Mantel Cox test). Data shown are representative of 3 experiments.
CD95 surface expression is significantly reduced on activated T cells from Toso-deficient mice compared with wt cells (data not shown), preventing proper analysis of CD95-mediated apoptosis in Toso-deficient T cells. Toso-deficient T cells do, however, express normal levels of TNFR-1 and TNFR-2. Because our data demonstrate that Toso promotes TNF-mediated prosurvival signals in human T cells, we thus further investigated the function of Toso in TNFα-induced T cell activation and apoptosis using Toso-deficient mice. In naive T cells, TNFα-engagement maintains cellular survival and enhances T-cell activation, while in activated T cells sustained TNF ligation leads to apoptosis.30,31 As TNF acts synergistically with low concentrations of IL-2 to induce T-cell activation, the potential role of Toso in TNFinduced T-cell activation was determined by stimulating freshly isolated naive CD8+ T cells from wt control mice or Toso−/− mice with TNFα for 12 hours in the presence of a low dose of IL-2 (2 ng/mL). T-cell activation was then determined by the up-regulation of the early T-cell activation marker CD69. Treatment with TNFα induced CD69 expression on a substantial proportion of wt CD8+ T cells. However, TNFinduced CD69 expression was significantly reduced in Toso-deficient CD8+ T cells (Figure 6B).
Next, we examined the ability of TNF to induce apoptosis in activated CD8+ T cells. T-cell blasts were generated by stimulating CD8+ T cells with CD3 and CD28. Exposure of these T cells blasts to 20 ng/mL TNF resulted in the induction of apoptotic cell death. However, as shown in Figure 6C, Toso−/− T-cell blasts exhibited substantially higher rates of apoptosis in comparison to Toso+/+ T cell blasts on TNF-engagement.
Thus, Toso deficiency results in increased sensitivity to TNF-induced apoptosis, as well as reduced TNF-mediated coactivation in CD8+ T cells, further underscoring an antiapoptotic/prosurvival function of Toso in death receptor signaling.
To investigate the biologic function of Toso in death receptor signaling in vivo, the effect of Toso-deficiency on TNF-mediated liver injury was examined. Injection of TNF plus the transcriptional inhibitor D(+)-galactosamine (GalN) causes acute liver injury in animals, which is mainly mediated by invariant natural killer T (iNKT) cells and recruitment of circulating lymphocytes into the hepatic sinus.34-36 Using the TNF/GalN model of liver injury, Toso−/− mice exhibited markedly reduced signs of liver injury in comparison to Toso+/+ mice, as assessed by measurement of alamine aminotransferase (ALT) levels in the serum (Figure 6D). Moreover, overall mortality in wt C57BL/6 mice was 72% within 20 hours after TNFα/GalN-administration, while in Toso−/− mice, TNFα/GalN-induced mortality was only 14% (Figure 6E). Thus, Toso−/− mice were largely protected from TNFα/GalN-induced lethal consequences.
Discussion
The death receptors TNFR1 and CD95 have multifunctional properties and can transmit both, death inducing as well as activating signals. However, the molecular elements that regulate proapoptotic versus prosurvival signaling of these receptors are still poorly defined. Here we demonstrate that the transmembrane protein Toso acts as a novel regulator of TNFR1 and CD95 signaling by facilitating death receptor–induced prosurvival signaling cascades.
Toso is a novel regulator of CD95L- and TNFα-induced signaling
To analyze the biologic function of Toso, we modulated the expression levels of Toso by specific gene knock-down, overexpression and also used Toso deficient mice. Our experiments revealed that the expression of Toso inhibits CD95-induced apoptosis in a dose dependent fashion. The antiapoptotic effects of Toso were observed in various tumor cell lines, as well as in primary T lymphocytes. Importantly, Toso acts not only on CD95-mediated signaling, but also exhibits a protective function on TNFα-mediated apoptosis and necroptosis, as well as on TRAIL-R–induced cell death. Furthermore, our data indicate that Toso is also critically involved in the regulation of TNFα-induced activatory signals, such as the activation of Erk and NF-κB. Together with previous studies that have reported protective functions of Toso in overexpression and transgenic systems,10,19 these data solidly establish an antiapoptotic function of Toso in CD95L and TNFα signaling and strongly suggest that Toso serves as a general regulator of death receptor signaling.
Toso facilitates RIP1 ubiquitination
As previous studies have reported a requirement of the adaptor kinase RIP1 for caspase independent necrotic cell death induced by CD95 and TNFRI,16-18 we hypothesized that Toso might use RIP1 as an effector molecule. In agreement with this concept, Toso was constitutively associated with RIP1 in Toso overexpressing cells and the inhibitory activity of Toso was largely abolished in RIP1 knock-down or null mutant cells. RIP1 was initially discovered in a yeast 2-hybrid screen as an interaction partner of CD95,29 its role in CD95-mediated apoptosis is, however, still controversial.23 RIP1 also binds to other death receptors and death domain containing adaptors and in TNF-receptor signaling has been demonstrated to be involved in the activation of NF-κB signaling pathways. Interestingly, the kinase domain of RIP1 is not required to mediate NF-κB activation by TNFα.26 Using a RIP1-K377R mutant, TNF-mediated activation of IKK has however recently been demonstrated to require site-specific ubiquitination of RIP1 at K377.23 CD95-stimulation also transiently induced RIP1 ubiquitination, which was dependent on Toso expression. Whether ubiquitinated RIP1 is subsequently targeted for proteosomal degradation is currently unclear. Furthermore, the RIP1-K377R mutant efficiently suppressed the antiapoptotic function of Toso. Hence, our studies strongly suggest that the antiapoptotic mechanism of Toso function involves ubiquitination of RIP1 at K377.
Our studies indicate that on CD95 stimulation Toso, RIP1 and FADD form a trimolecular complex. This observation extends previous reports describing an interaction between the death domain of RIP1 and FADD,29 and an in vitro interaction between FADD and the C-terminus of Toso.19 While Toso and RIP1 interact constitutively with each other, the association of FADD with the complex is stabilized by CD95-stimulation. Furthermore, our studies using the RIP1-K377R ubiquitination mutant indicate that efficient recruitment of FADD also depends on RIP1 ubiquitination. While the exact molecular mechanism that couples the Toso/RIP1 protein complex to the CD95 signaling machinery is still not completely resolved, our current working hypothesis is, that Toso facilitates the ubiquitination of RIP1 on lysine 377 on CD95 stimulation. Analogous to TNF receptor signaling, RIP1 ubiquitination may involve the E3 ligases cIAP1/2 and TRAF2.24,25 Ubiquitinated RIP1 can then recruit FADD to form a trimolecular protein complex consisting of Toso, RIP1 and FADD. The formation of this protein complex may serve a dual function. First, this complex may serve as a molecular sink for the death adaptor FADD, which then would not be available for efficient apoptotic signal propagation at activated CD95 receptors, thereby decreasing apoptosis. Second, Toso and ubiquitinated RIP1 may exhibit an additional antiapoptotic function by activating cellular prosurvival signals. Similar functions have been described for ubiquitinated RIP1 in the TNFα system.22-24
Toso promotes death receptor–induced nonapoptotic signaling
Our data demonstrate that Toso expression promotes the activation of death-receptor–mediated prosurvival signals in response to both, CD95L as well as TNFα. In Toso overexpressing cells, which exhibit reduced sensitivity to CD95-induced cell death, CD95 ligation induces Erk1/2 activation. Conversely, in Toso knock-down cells TNFα-mediated activation of NF-κB is significantly reduced. Furthermore, Toso−/− CD8+ T cells are more sensitive to TNFα-induced apoptosis and show reduced TNFα-mediated coactivation. Together, these observations suggest that Toso exhibits its antiapoptotic effects by supporting death receptor–mediated cellular activation pathways. Although the prototypic death receptor CD95 is primarily recognized as a death-inducing receptor, accumulating evidence indicates that CD95 ligation can also induce nonapoptotic signaling pathways.6-9,27 Similarly, TNFR1 is well characterized for its proinflammatory activities, but also acts as a death inducing receptor in the immune system. It has been proposed that death receptor stimulation may simultaneously induce apoptotic and nonapoptotic signals in all cells to some extent. The balance of pro- versus antiapoptotic signals then defines the specific cellular response induced by death receptor stimulation. Our findings on the novel antiapoptotic mechanism of Toso provide a molecular basis for CD95L and TNFα-induced activation of prosurvival signals. We propose that as a result of the relative augmentation of survival signals versus apoptotic signals, Toso raises the threshold for death receptor–mediated apoptosis.
Pathophysiologic functions of Toso
The role of TNFα in liver pathology has been studied extensively. Using the well established TNFα/GalN model of experimental hepatitis, we could demonstrate that Toso−/− mice were largely resistant to TNFα-induced liver injury and protected from TNFα-induced lethal consequences. This protection from TNF-induced liver damage and lethality in Toso deficient mice reveals an important physiologic function of Toso in TNFα-signaling.
Given an antiapoptotic/prosurvival function of Toso, these results may appear counterintuitive at first sight. However, recent findings have demonstrated that the liver-damaging effects of TNFα in this disease model are largely mediated indirectly via the activation iNKT cells.33-35 In addition, TNFα mediates an increased influx of circulating lymphocytes into the hepatic sinus and their subsequent local proliferation via blastoid formation, which further contributes to liver destruction.33,34 The high expression levels of Toso in NKT cells and in activated cytotoxic T cells, together with a lack of Toso expression in hepatocytes therefore suggests the following scenario. On TNFα-engagement Toso−/− leukocytes are more likely to undergo apoptosis and only few potentially autodestructive immune cells get activated in Toso−/− mice. In this way, Toso−/− mice are largely protected from TNFα-mediated liver damage.
The reverse may apply for autoimmunity and certain types of cancer, where an up-regulation of Toso expression is frequently observed. The overexpression of Toso in chronic lymphocytic leukemia (CLL) is associated with progressive disease.13,14 It is tempting to speculate that the increased level of Toso expression in leukemia cells renders these cells resistant to CD95-induced apoptosis. Whether Toso may promote nonapoptotic signals through CD95 under these pathologic conditions is an exciting possibility that should be examined. Furthermore, future studies might reveal novel ligands for Toso that potentially could modulate its antiapoptotic activity. This idea should also be considered in light of the recent identification of Toso as a putative Fcμ-receptor,11 although in our experimental system we did not detect significant binding of IgM to Toso. Thus, the identification and characterization of physiologic Toso ligands is an interesting topic that awaits further investigation.
Importantly, the antiapoptotic function of Toso, as well as TNFα-mediated activation of MAPKs could be blocked by a Toso specific monoclonal antibody (mAb A38). These findings may lead to the development of novel therapeutic applications for the treatment of leukemias, and other immunopathologic disorders. By using Toso blocking antibodies it may be possible to overcome resistance to death receptor–mediated apoptosis in Toso overexpressing autoreactive and/or leukemia cells. In other therapeutic applications, Toso blocking antibodies may protect from TNFα-induced tissue destruction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are very grateful to Dr Adrian Ting and to Dr Brian Seed for providing plasmids for RIP1 wt and K377R-RIP1 and RIP1−/− Jurkat, respectively.
This work was supported by grants from the Fritz Thyssen Research Foundation (Az. 10.08.2.155), the German Research Foundation (DFG: LE 1254/2-1), and the Marie Curie International Reintegration Grant (FP7-202287).
Authorship
Contribution: X.N., P.L., K.L, D.A., G.F., N.F., M.A.K., P.P., S.M., and C.W. performed research and analyzed data; T.M. and A.C.C. contributed reagents and mice; and K.L. and N.F. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: A.C.C. is an employee of Genentech Inc. The remaining authors declare no other competing financial interests.
Correspondence: Kyeong-Hee Lee, Research Center Borstel, Leibniz Center for Medicine and Biosciences, Dept of Immunology and Cell Biology, Parkallee 22, 23845 Borstel, Germany; e-mail: klee@fz-borstel.de.
References
Author notes
X.-H.N., P.A.L., K.S.L., D.A., and G.F. contributed equally to this paper.