Abstract
The prognosis of acute myeloid leukemia (AML) is very poor in elderly patients, especially in those classically defined as having unfavorable cytogenetics. The recent monosomal karyotype (MK) entity, defined as 2 or more autosomal monosomies or combination of 1 monosomy with structural abnormalities, has been reported to be associated with a worse outcome than the traditional complex karyotype (CK). In this retrospective study of 186 AML patients older than 60 years, the prognostic influence of MK was used to further stratify elderly patients with unfavorable cytogenetics. CK was observed in 129 patients (69%), and 110 exhibited abnormalities according to the definition of MK (59%). MK+ patients had a complete response rate significantly lower than MK− patients: 37% vs 64% (P = .0008), and their 2-year overall survival was also decreased at 7% vs 22% (P < .0001). In multivariate analysis, MK appeared as the major independent prognostic factor related to complete remission achievement (odds ratio = 2.3; 95% confidence interval, 1-5.4, P = .05) and survival (hazard ratio = 1.7; 95% confidence interval, 1.1-2.5, P = .008). In the subgroup of 129 CK+ patients, survival was dramatically decreased for MK+ patients (8% vs 28% at P = .03). These results demonstrate that MK is a major independent factor of very poor prognosis in elderly AML.
Introduction
Major advances in the treatment of adult patients with acute myeloid leukemia (AML) have been obtained with intensive postremission therapies, including high-dose chemotherapy and/or allogeneic hematopoietic stem cell transplantation.1-3 However, AML occurs more frequently after the age of 60 years, and such options are not adapted for elderly patients because they frequently present a poor clinical condition or severe comorbidities. Treatment results remain unsatisfactory even in the cohort of older AML patients eligible for intensive chemotherapy for 2 main reasons: (1) an increased incidence of adverse disease-related factors, such as an unfavorable karyotype, which may explain a low complete remission (CR) rate and a short response duration; and (2) suboptimal postremission treatment compared with younger adults because these patients cannot be offered repeated consolidation courses with high-dose chemotherapy.4
Cytogenetic features of the blasts at diagnosis have been identified as a major prognostic factor in AML in all age groups, leading to the AML cytogenetic classification.5-7 A complex karyotype (CK), described as the combination of multiple structural abnormalities, was demonstrated as unfavorable, associated with a poor outcome.5,8 CK was first defined by the Medical Research Council AML Working Group as the combination of at least 5 cytogenetic abnormalities,7 but a Cancer and Leukemia Group B study showed subsequently a similar prognostic influence whether CK was defined by ≥ 3, 4, or 5 abnormalities.5 Several studies have investigated the prognostic impact of cytogenetics in elderly AML patients. The Medical Research Council assessed the cytogenetic classification in a cohort of 1065 patients older than 55 years.9 Almost 20% of these patients had abnormalities classifying them in the unfavorable group, and their outcome strongly correlated with cytogenetics.9 Subsequently, the Eastern Cooperative Oncology Group,10 the Cancer and Leukemia Group B,11 and the German Austrian AML Study Group12 also analyzed the impact of cytogenetics on the CR rate and survival in cohorts of patients older than 55 or 60 years and showed cytogenetics to be a strong and independent prognostic factor in multivariate analysis11,12 (together with age and/or leukocytosis).
In 2008, Breems et al proposed the concept of monosomal karyotype (MK), defined by the presence of at least 2 autosomal monosomies or 1 monosomy plus 1 or more structural abnormalities.13 In 733 AML patients younger than 60 years with cytogenetic abnormalities, MK was shown to be associated with a very poor prognosis and a more powerful prognostic predictor than CK. MK status was not investigated in elderly patients in this study. The recent large Southwest Oncology Group (SWOG) study included a subgroup of 457 elderly AML patients older than 60 years.14 In this subgroup, 90 patients had an MK that appeared to be associated with a shorter survival.
Because elderly AMLs are characterized by a higher prevalence of poor-risk cytogenetic abnormalities than adult AML,15 with less favorable abnormalities (core binding factor type), more frequent monosomies and comparatively less abnormalities, such as t(6;9), 3q abnormalities or 11q23/MLL abnormalities, an improvement in the prognostic stratification of cytogenetic subgroups might be of particular interest to optimize therapeutic decisions. The identification of a very poor-risk cytogenetic subgroup should lead in elderly patients to consider an alternative therapeutic approach, either experimental or palliative, rather than standard intensive chemotherapy.
To further investigate the incidence, features, and specific prognostic relevance of MK in this elderly population, we conducted on behalf of the Groupe Ouest Est des Leucémies Aiguës et Autres Maladies du Sang (GOELAMS) a retrospective study of 186 AML patients older than 60 years of age with classically defined unfavorable cytogenetics AML.
Methods
Patients and treatment protocols
Eligibility for this study was limited to patients with previously untreated AML and unfavorable cytogenetics, enrolled between 1996 and 2006 (after obtaining written consent according to the Declaration of Helsinki) in 1 of 3 successive prospective trials focused on AML in elderly patients (older than 60 years). Standard intensive chemotherapy with cytarabine and idarubicin was used in all 3 trials for induction. Postremission treatment was composed of monthly or quarterly reinduction courses associated with maintenance chemotherapy. Treatment schedules of the 3 trials are presented in Figure 1. Of note, poor cytogenetics patients were well balanced between the treatment arms.
The SA4 GOELAMS study was designed to directly compare by randomization the potential effects of fludarabine given in association with Ara-C during induction and postremission treatment in patients 60 to 75 years of age with de novo AML.16 A total of 294 eligible patients were enrolled, including 63 with high-risk cytogenetics (24% of 260 patients with available data).
The SA2002 randomized trial, aimed to assess whether the addition of androgens to postremission therapy, was associated with an improved outcome in elderly patients (≥ 60 years old) with de novo AML.17 This trial included 330 patients, 60 to 86 years of age. Eighty of 308 with available cytogenetics presented with high-risk AML. Leukemia-free survival and overall survival (OS) were improved in the androgen arm.
The R04 trial (43 patients) was a phase 2 study that assessed the combination of gemtuzumab ozogamycin with intensive induction chemotherapy and was restricted to patients 60 to 75 years of age with de novo or secondary AML and unfavorable karyotype.18
Together with the 43 patients enrolled in the R04 trial, this study included 186 patients, 143 being issued from the 568 AML patients with available cytogenetic data treated in the SA4 and SA2002 trials (25.2%).
The trial hypotheses tested changed neither the CR rate nor the 2-year OS, except for the favorable impact of androgen maintenance on the 5-year leukemia-free survival and OS in the SA2002 trial.17 This allowed us to compile data from different trials together in 1 cohort. This cohort was approved by the institutional review boards of all participating institutions.
Cytogenetic analyses
At diagnosis, bone marrow samples were provided to local cytogenetics laboratories of the various centers for karyotypic analyses. Standard banding techniques were used on the mitoses obtained. All cytogenetic data were centrally reviewed by the GOELAMS Cytogenetics Committee and annotated according to the International System for Human Cytogenetic Nomenclature.19 An abnormality was considered clonal when at least 2 metaphases had the same aberration in case of a structural abnormality or an extra chromosome. Monosomy had to be present in at least 3 metaphases to be considered significant. A minimum of 20 normal metaphases was required to define a normal karyotype. The presence of t(8;21) or inv(16)/t(16;16) defined the favorable group. Cytogenetic abnormalities defining the unfavorable group were −5/del(5q), −7/del(7q), 3q26/EVI1, t(6;9), t(9;22), 11q23/MLL [except t(9;11)], and complex rearrangements with 3 clonal abnormalities or more. The intermediate group included normal karyotype and other abnormalities. Karyotypes were further analyzed to delineate 3 categories, respectively, with no monosomy, 1 monosomy (associated or not with other structural abnormalities), and 2 or more monosomies. The MK status was assessed retrospectively according to the Breems et al definition: presence of at least 2 autosomal monosomies or 1 monosomy plus 1 structural abnormality.13
Evaluation of treatment
The efficacy of induction therapy was evaluated after 1 course: CR was defined according to Cheson's revised recommendations20 as a normocellular bone marrow containing < 5% blasts associated with a neutrophils count > 1 × 109/L and a platelets count > 100 × 109/L in peripheral blood. Response assessment did not rely on cytogenetics. Persistent leukemia was defined as a partial response or no response and mortality at induction by early death during the first 7 days of treatment or subsequent chemotherapy-induced hypoplasia.
Statistical analyses
OS was the main objective of the study. Secondary objectives were to evaluate CR and leukemia persistence rates. The Fisher exact test was used for comparison of binary variables between cytogenetic groups. OS was calculated from the time of inclusion until the date of death or last contact, and alive patients were censored at the time of last contact. Survival curves for OS were estimated by the Kaplan-Meier method, and comparisons were made by the log-rank test. In multivariate analyses, outcome comparisons were adjusted with the Cox model and tested by the likelihood ratio test. P values < .05 were considered of statistical significance. Hazard ratios were given with 95% confidence intervals (CIs). All calculations were performed using MedCalc Version 9.3 software.
Results
Patients' characteristics, overall outcome, and cytogenetic abnormalities
The median age in this high-risk cohort of 186 patients was 68 years (range, 60-79 years), and the male/female ratio was 1.04. A majority of patients (176 of 186) presented de novo AML. Ten of 43 patients (23%) enrolled in R04 trial had secondary AML.
Of these 186 patients assessable for postinduction response and survival, 90 (48%) achieved CR. There were 31 deaths (17%) during induction course, and 65 patients (35%) showed persistent leukemia. After a median follow-up of 43 months for survivors, the OS was 13.7% at 2 years (95% CI, 12.4%-14.9%). The CR rate was not significantly different between patients 60 to 69 years of age and patients older than 69 years: 46% and 51%, respectively (P = .6). There also was no significant difference in 2-year OS between these 2 age categories (P = .87). There was no influence of the treatment protocol for either CR (P = .17) or 2-year OS rate (P = .24).
Distribution of the different unfavorable cytogenetic abnormalities among the 186 patients and their relationship to outcome are summarized in Table 1. Poor cytogenetic features included monosomy 5 or del(5q) in 85 patients (46%) and monosomy 7 or del(7q) in 76 (41%). Only 9 patients (4.8%) had 11q23/MLL abnormalities, 15 (8%) 3q21q26 abnormalities, and 3 patients presented a t(6;9) translocation. A CK as defined by 3 or more clonal abnormalities was noted in 129 patients (69%). CR rates ranged from 41% in patients with del(5q) to 53% in patients with 3q abnormalities and 2-year OS between 6% in patients with monosomy 5 and 16% in patients with del(7q). Patients with CK had lower rates of CR and 2-year OS at 39% and 12%, compared with patients without CK (CR rate at 68% and 2-year OS at 17%, P < .002 and P < .005, respectively).
Distribution of unfavorable cytogenetic abnormalities among the 186 patients, CR rate, and outcome
| Cytogenetic abnormalities . | N . | CR rate (95% CI) . | 2-year OS (95% CI) . |
|---|---|---|---|
| 3q abnormalities | 15 | 53% (25-81) | 7% (1-13) |
| del(5q) | 55 | 41% (28-54) | 7% (4-10) |
| −5 | 30 | 43% (25-61) | 6% (2.5-9.5) |
| del(7q) | 30 | 50% (32-68) | 16% (12-20) |
| −7 | 46 | 48% (33-63) | 12% (6-18) |
| 11q23/MLL except t(9;11) | 9 | 33% (0-83) | 11% (1-21) |
| t(6;9) | 3 | 100% (0-100) | 33% (6-60) |
| CK (≥ 3 abnormalities) | 129 | 39% (29-49) | 12% (9-15) |
| CK5 (≥ 5 abnormalities) | 91 | 38% (28-48) | 6% (3.5-8.5) |
| Cytogenetic abnormalities . | N . | CR rate (95% CI) . | 2-year OS (95% CI) . |
|---|---|---|---|
| 3q abnormalities | 15 | 53% (25-81) | 7% (1-13) |
| del(5q) | 55 | 41% (28-54) | 7% (4-10) |
| −5 | 30 | 43% (25-61) | 6% (2.5-9.5) |
| del(7q) | 30 | 50% (32-68) | 16% (12-20) |
| −7 | 46 | 48% (33-63) | 12% (6-18) |
| 11q23/MLL except t(9;11) | 9 | 33% (0-83) | 11% (1-21) |
| t(6;9) | 3 | 100% (0-100) | 33% (6-60) |
| CK (≥ 3 abnormalities) | 129 | 39% (29-49) | 12% (9-15) |
| CK5 (≥ 5 abnormalities) | 91 | 38% (28-48) | 6% (3.5-8.5) |
Incidence and prognostic influence of autosomal monosomies
At least 1 autosomal monosomy was observed in 119 patients (64%; Table 2). The most frequent monosomies were −7 (n = 46) and −5 (n = 30) followed by −17, −16, and −18. These patients had a CR rate of 39% and a 2-year OS of 8%.
Incidence of autosomal monosomies and prognostic effect of combination with other chromosomal structural abnormalities
| Type of monosomy . | No. of patients with autosomal chromosomal monosomy . | No. of patients with 1 monosomy without other structural abnormality . | No. of patients with 1 monosomy and at least 1 structural abnormality . | No. of patients with 2 or more autosomal monosomies . |
|---|---|---|---|---|
| −1 | 0 | 0 | 0 | 0 |
| −2 | 3 | 0 | 0 | 3 |
| −3 | 9 | 0 | 0 | 9 |
| −4 | 8 | 0 | 0 | 8 |
| −5 | 30 | 1 | 1 | 28 |
| −6 | 4 | 0 | 0 | 4 |
| −7 | 46 | 6 | 13 | 27 |
| −8 | 7 | 0 | 2 | 5 |
| −9 | 5 | 0 | 0 | 5 |
| −10 | 6 | 0 | 1 | 5 |
| −11 | 11 | 0 | 1 | 10 |
| −12 | 12 | 0 | 0 | 12 |
| −13 | 11 | 0 | 1 | 10 |
| −14 | 9 | 0 | 1 | 8 |
| −15 | 14 | 0 | 0 | 14 |
| −16 | 23 | 0 | 1 | 22 |
| −17 | 30 | 1 | 1 | 28 |
| −18 | 22 | 0 | 1 | 21 |
| −19 | 5 | 0 | 0 | 5 |
| −20 | 15 | 0 | 1 | 14 |
| −21 | 14 | 1 | 1 | 12 |
| −22 | 10 | 0 | 1 | 9 |
| Total no. (%) of patients | 119 (64) | 9 (5) | 26 (14) | 84 (45) |
| CR rate | 39% | 77% | 40% | 37% |
| 95% CI | 32-46 | 56-98 | 26-54 | 27-47 |
| 2-year OS rate | 8% | 18% | 7% | 7% |
| 95% CI | 5.5-10.5 | 13.5-22.5 | 3-11 | 4-10 |
| Type of monosomy . | No. of patients with autosomal chromosomal monosomy . | No. of patients with 1 monosomy without other structural abnormality . | No. of patients with 1 monosomy and at least 1 structural abnormality . | No. of patients with 2 or more autosomal monosomies . |
|---|---|---|---|---|
| −1 | 0 | 0 | 0 | 0 |
| −2 | 3 | 0 | 0 | 3 |
| −3 | 9 | 0 | 0 | 9 |
| −4 | 8 | 0 | 0 | 8 |
| −5 | 30 | 1 | 1 | 28 |
| −6 | 4 | 0 | 0 | 4 |
| −7 | 46 | 6 | 13 | 27 |
| −8 | 7 | 0 | 2 | 5 |
| −9 | 5 | 0 | 0 | 5 |
| −10 | 6 | 0 | 1 | 5 |
| −11 | 11 | 0 | 1 | 10 |
| −12 | 12 | 0 | 0 | 12 |
| −13 | 11 | 0 | 1 | 10 |
| −14 | 9 | 0 | 1 | 8 |
| −15 | 14 | 0 | 0 | 14 |
| −16 | 23 | 0 | 1 | 22 |
| −17 | 30 | 1 | 1 | 28 |
| −18 | 22 | 0 | 1 | 21 |
| −19 | 5 | 0 | 0 | 5 |
| −20 | 15 | 0 | 1 | 14 |
| −21 | 14 | 1 | 1 | 12 |
| −22 | 10 | 0 | 1 | 9 |
| Total no. (%) of patients | 119 (64) | 9 (5) | 26 (14) | 84 (45) |
| CR rate | 39% | 77% | 40% | 37% |
| 95% CI | 32-46 | 56-98 | 26-54 | 27-47 |
| 2-year OS rate | 8% | 18% | 7% | 7% |
| 95% CI | 5.5-10.5 | 13.5-22.5 | 3-11 | 4-10 |
All monosomies (isolated or associated) are taken into account, explaining that the total number of monosomies exceeds the total number of patients.
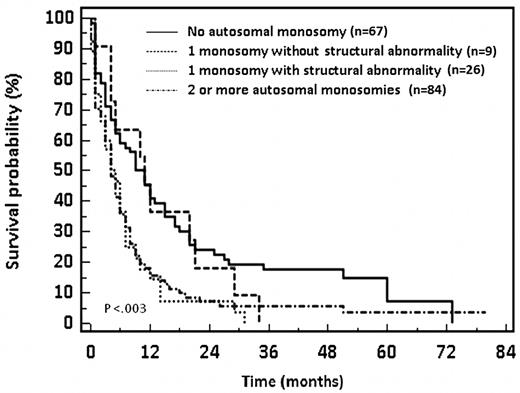
The outcome was more favorable in patients showing a single monosomy without other structural chromosomal abnormality (n = 9) with a 77% CR rate and 2-year OS of 18%. Conversely, patients with either 1 monosomy associated with at least 1 structural abnormality or with 2 or more monosomies had a lower CR rate (37%-40%) and a reduced 2-year OS (7%). These differences in outcome are statistically significant (P < .004 and P < .003, respectively). Survival data are shown in Figure 2. Of note, the 2-year OS of patients with a single monosomy without structural chromosomal abnormality was similar to that of patients without autosomal monosomy (18% and 22%, respectively, P = .79).
OS of patients with unfavorable cytogenetics AML in relation to the number and type of autosomal chromosomal monosomies.
OS of patients with unfavorable cytogenetics AML in relation to the number and type of autosomal chromosomal monosomies.
Patients' characteristics and outcome according to the MK status
Of of the 186 patients with unfavorable cytogenetics in this cohort, 110 (59%) had abnormalities in agreement with MK criteria as defined by Breems et al.13 Patient characteristics of both MK-negative (MK−) and MK-positive (MK+) groups are summarized in Table 3. The proportion of MK+ patients was similar in the different trials (P = .3). The median age of MK− and MK+ patients was similar at 68 years (P = .2). Only 5 of 10 patients with secondary AML presented an MK. The incidence of hyperleukocytosis over 30 × 109/L was similar in MK− and MK+ groups at 17% and 14%, respectively (P = .7).
Relationship between MK status and other prognostic factors
| . | Absence of MK (MK− group) . | Presence of MK (MK+ group) . | ||||
|---|---|---|---|---|---|---|
| No. of patients . | CR rate (95% CI) . | 2-year OS (95% CI) . | No. of patients . | CR rate (95% CI) . | 2-year OS (95% CI) . | |
| Total cohort | 76 | 64% (53-75) | 22% (17.5-26.5) | 110 | 37% (28-46) | 7% (4.5-9.5) |
| Age groups | ||||||
| 60-69 y | 46 | 58% (43-73) | 21% (15-27) | 62 | 37% (25-49) | 7% (3.5-10.5) |
| 70-79 y | 30 | 73% (56-90) | 26% (18-34) | 48 | 37% (23-51) | 7% (3-11) |
| Type of leukemia | ||||||
| De novo AML | 71 | 63% (51-75) | 23% (16-30) | 105 | 39% (29-49) | 8% (5-11) |
| Secondary AML | 5 | NA | NA | 5 | NA | NA |
| Leukocytosis | ||||||
| WBC < 30 | 63 | 65% (53-77) | 25% (20-30) | 95 | 38% (28-48) | 6% (3.5-8.5) |
| WBC ≥ 30 | 13 | 61% (30-92) | 15% (5-25) | 15 | 26% (2-50) | 13% (5-21) |
| Complex karyotype | ||||||
| ≥ 3 abnormalities | 29 | 44% (24-64) | 28% (20-36) | 100 | 38% (29-47) | 8% (5.5-10.5) |
| ≥ 5 abnormalities | 9 | 33% (5-61) | 22% (9-35) | 82 | 39% (28-50) | 4% (2-6) |
| Other unfavorable abnormalities | ||||||
| −5 | 0 | NA | NA | 30 | 43% (24-62) | 6% (2-10) |
| del(5q) | 22 | 59% (36-82) | 18% (10-26) | 33 | 30% (13-47) | 0% (0-0) |
| −7 | 6 | 83% (43-100) | 16% (1-31) | 40 | 42% (26-58) | 11% (6-16) |
| del(7q) | 14 | 57% (27-87) | 21% (10-32) | 16 | 43% (16-70) | 12% (4-20) |
| 3q21q26 | 7 | 71% (23-100) | 14% (1-27) | 8 | 38% (0-89) | 0% (0-0) |
| 11q23/MLL | 4 | NA | NA | 5 | NA | NA |
| t(6;9) | 3 | NA | NA | 0 | NA | NA |
| . | Absence of MK (MK− group) . | Presence of MK (MK+ group) . | ||||
|---|---|---|---|---|---|---|
| No. of patients . | CR rate (95% CI) . | 2-year OS (95% CI) . | No. of patients . | CR rate (95% CI) . | 2-year OS (95% CI) . | |
| Total cohort | 76 | 64% (53-75) | 22% (17.5-26.5) | 110 | 37% (28-46) | 7% (4.5-9.5) |
| Age groups | ||||||
| 60-69 y | 46 | 58% (43-73) | 21% (15-27) | 62 | 37% (25-49) | 7% (3.5-10.5) |
| 70-79 y | 30 | 73% (56-90) | 26% (18-34) | 48 | 37% (23-51) | 7% (3-11) |
| Type of leukemia | ||||||
| De novo AML | 71 | 63% (51-75) | 23% (16-30) | 105 | 39% (29-49) | 8% (5-11) |
| Secondary AML | 5 | NA | NA | 5 | NA | NA |
| Leukocytosis | ||||||
| WBC < 30 | 63 | 65% (53-77) | 25% (20-30) | 95 | 38% (28-48) | 6% (3.5-8.5) |
| WBC ≥ 30 | 13 | 61% (30-92) | 15% (5-25) | 15 | 26% (2-50) | 13% (5-21) |
| Complex karyotype | ||||||
| ≥ 3 abnormalities | 29 | 44% (24-64) | 28% (20-36) | 100 | 38% (29-47) | 8% (5.5-10.5) |
| ≥ 5 abnormalities | 9 | 33% (5-61) | 22% (9-35) | 82 | 39% (28-50) | 4% (2-6) |
| Other unfavorable abnormalities | ||||||
| −5 | 0 | NA | NA | 30 | 43% (24-62) | 6% (2-10) |
| del(5q) | 22 | 59% (36-82) | 18% (10-26) | 33 | 30% (13-47) | 0% (0-0) |
| −7 | 6 | 83% (43-100) | 16% (1-31) | 40 | 42% (26-58) | 11% (6-16) |
| del(7q) | 14 | 57% (27-87) | 21% (10-32) | 16 | 43% (16-70) | 12% (4-20) |
| 3q21q26 | 7 | 71% (23-100) | 14% (1-27) | 8 | 38% (0-89) | 0% (0-0) |
| 11q23/MLL | 4 | NA | NA | 5 | NA | NA |
| t(6;9) | 3 | NA | NA | 0 | NA | NA |
NA indicates not applicable; and WBC, white blood count.
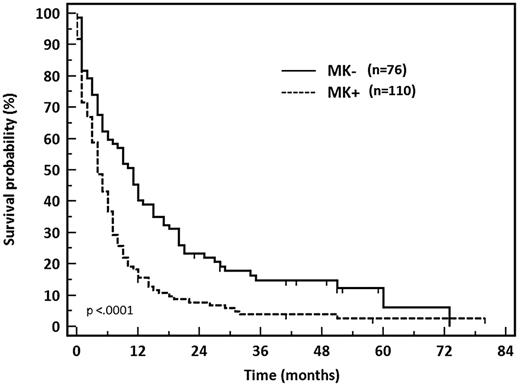
MK+ patients had a significantly lower CR rate at 37% (41 of 110) than MK− patients (64%, 49 of 76, P = .0008). The 2-year OS was also significantly impaired in MK+ patients at 7% vs 22% (P < .0001; Figure 3). For comparison, results observed in other cytogenetic subgroups when considering the 538 patients of the SA4 and SA 2002 trials were as follows: CR rates 83%, 74%, and 60% and 2-year OS 70%, 45%, and 40% in patients with favorable (n = 23), normal (n = 280), and intermediate karyotype (n = 92), respectively.
OS of patients with unfavorable cytogenetics AML according to MK status.
MK+ patients had a quite similar CR rate at 37% and 2-year OS rate at 7% whether they were 60 to 69 years of age or 70 to 79 years of age (P = .45). The outcome of MK− patients was not significantly different in these 2 age categories in terms of CR rate or 2-year OS (58% vs 73% for CR, 21% vs 26% for OS, respectively, P = .09 and P = .46).
All patients with monosomy 5 belonged to the MK+ group and had a 43% CR rate and an average 2-year OS of 6%. The CR rate was lower in MK+ patients with del(5q) (30% vs 59%) and 2-year OS was abysmal (0% vs 18%).
Prognostic influence of MK compared with CK
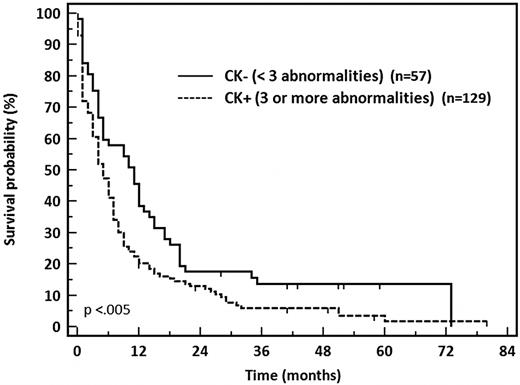
CK was confirmed to confer a negative prognostic impact. The CR rate was significantly lower in CK+ patients, at 39% (51 of 129) compared with 68% (39 of 57) in CK− patients (P = .002). The 2-year OS also was significantly decreased in patients with CK, at 12% vs 17% in patients with poor-risk CK− AML (P < .005; Figure 4). CK defined by 5 or more abnormalities (CK5) was also a predictive factor for very poor prognosis in this cohort. The 2-year OS was dramatically impaired in patients with CK5 at 6% vs 22% for other poor-risk AML patients (P < .0002). The more recently described21 threshold of CK4 (n = 111) showed intermediate results with a CR rate of 39% and OS of 11% significantly different compared with CK4− patients who displayed a CR rate of 63% and 19% OS at 2 years (P = .003).
OS of patients with unfavorable cytogenetics AML according to CK status discriminated on 3 or more abnormalities.
OS of patients with unfavorable cytogenetics AML according to CK status discriminated on 3 or more abnormalities.
The majority of the 110 patients with MK also had a CK. CK+ patients experienced a very poor outcome when they also belonged to the MK+ group: CR rate, 38% vs 44% (P = .41) and 2-year OS, 8% vs 28%, respectively (P = .03). There were discrepancies between MK and CK presentations in 39 patients. Ten patients had MK without CK (MK+CK− group) and experienced the same very poor prognosis as other patients with MK (2-year OS, 0% and 8%, P = .59). Conversely, 29 patients had CK without MK (MK−CK+ group) and had a similar outcome as all other patients without MK (2-year OS, 28% and 22%, P = .8).
With regard to the definition of CK5, 28 patients belonged to the MK+CK5− group and 9 patients were MK−CK5+. Although there was a trend for a better outcome for these patients not cumulating both risk factors, this was not statistically significant, probably because of the small size of the subgroups.
In multivariate analysis using a Cox model, including MK and CK variables only (because age, leukocytosis, and treatment arm had no significant influence in univariate analysis), MK appeared as the major independent prognostic factor for CR (odds ratio = 2.3; 95% CI, 1-5.4, P = .05) and for OS (hazard ratio = 1.70; 95% CI, 1.1-2.5, P = .008), as summarized in Table 4. Comparison with CK, MK appeared to be a more robust predictor of CR and OS.
Multivariate analysis
| . | Variable . | Odds ratio/hazard ratio . | 95% CI . | P . |
|---|---|---|---|---|
| CR | Complex karyotype | 1.9 | 0.8-5 | .14 |
| Monosomal karyotype | 2.3 | 1-5.4 | .05 | |
| OS | Complex karyotype | 1.1 | 0.7-1.7 | .65 |
| Monosomal karyotype | 1.7 | 1.1-2.5 | .008 |
| . | Variable . | Odds ratio/hazard ratio . | 95% CI . | P . |
|---|---|---|---|---|
| CR | Complex karyotype | 1.9 | 0.8-5 | .14 |
| Monosomal karyotype | 2.3 | 1-5.4 | .05 | |
| OS | Complex karyotype | 1.1 | 0.7-1.7 | .65 |
| Monosomal karyotype | 1.7 | 1.1-2.5 | .008 |
Discussion
The negative prognostic impact of autosomal monosomies on AML outcome, especially of MK, was first demonstrated by Breems et al in a large cohort of adult patients younger than 60 years.13 This description has been recently confirmed by the SWOG in a study that included patients between 16 and 88 years of age.14
The present study analyzed the prognostic influence of MK in a cohort composed exclusively of elderly patients (older than 60 years) with unfavorable cytogenetics AML. To test a large and homogeneous cohort of patients, we restricted our analysis to patients with an unfavorable karyotype. The cohort could therefore also include the 43 patients enrolled in the RO4 trial, which had been designed for high-risk patients only. On the other hand, MK is a very rare event outside the context of unfavorable AML in the elderly: only 1 patient of 568 with available cytogenetic data enrolled in SA4 and SA2002 trials showed an MK without any associated criterion for unfavorable karyotype. Our results suggest that the definition of MK is well adapted also in elderly AML because elderly patients with no monosomy or a single monosomy without other structural abnormality experienced a better outcome than those with MK. This result is consistent with the observations from Breems et al in adult AML patients.13 Moreover, the high incidence of cytogenetic abnormalities and particularly monosomies must be underlined in elderly AML. The incidence of MK is indeed higher in this population than in younger adult AML patients: 16% of the 568 elderly patients included in SA4 and SA2002 trials in our study vs 9.5% (184 of 1975) of adult patients in the Breems et al study.13 The SWOG also showed that the proportion of MK+ patients increased with age: 4% in patients younger than 30 years, 12% in patients between 30 and 60 years of age, and 20% in patients older than 60 years.14
The influence of MK on treatment outcome was at least as important in our elderly cohort as in younger adult patients: here, we demonstrated that MK was associated with a significantly lower CR rate, at 37% vs 64%, and a significantly impaired 2-year OS, at 7% vs 22%. By comparison, the 4-year OS was significantly decreased at 4% vs 21% in the MK+ group of the Breems et al adult cohort13 and at 3% vs 13% in the SWOG study.14 Although these 3 studies were performed at different times and with different schedules, these figures are amazingly similar, underscoring the relevance of the prognostic value of MK. Our results might even suggest that the MK definition is more adapted to elderly than adult AML patients because the prognostic influence of MK overcomes in our elderly cohort the impact of other classic prognostic factors, such as age, leukocytosis, and classic poor-risk cytogenetic abnormalities, including CK. The MK status had a similar prognostic influence in our cohort of elderly patients without difference between the 2 age groups (60-70 years and > 70 years old). To note, MK+ was associated with a statistically significant decrease of OS in patients with del(5q): 2-year OS impaired at 0% in MK+ patients vs 18% in MK− (P = .0002). Our results were different from those of the whole cohort of the SWOG study, in which MK was not associated with an impaired OS in the subgroup with del(5q).
The most important observation of our study is that MK retains a prognostic impact within the subgroup of CK patients because 2-year OS was impaired to 8% in CK+MK+ patients compared with 28% in CK+MK− patients (P = .03). This observation in elderly patients is concordant with the Breems et al results in adult patients13 and with the results of the whole cohort of the SWOG.14 However, whereas CK remained an independent prognostic factor for OS in the latter (hazard ratio = 1.5; 95% CI, 1.1-1.9), MK is the only independent factor influencing CR rate and OS in our study (odds ratio = 2.3; 95% CI, 1.0-5.4; and hazard ratio = 1.7; 95% CI, 1.1-2.5).
In our study, CK and CK5 had a different prognostic impact, CK5 being a better indicator of patients with very poor outcome (12% OS at 2 years for CK+ patients and 6% for CK5+ patients). Regarding CK and CK5, Breems et al13 reported 4-year OS at 26% and 25% in CK+MK− and CK5+MK− patients, respectively, and 3% in both CK+MK+ and CK5+MK+ groups. We consider that MK is not only better than CK for stratifying elderly unfavorable AML patients but also better than CK5 because it applied to a higher number of patients in our cohort (110 vs 91 patients with MK and CK5, respectively).
Although caution should be exerted with such small series, it is interesting to note that, considering the 10 patients with MK but without CK (MK+CK−), 5 of them had a 3q21q26 abnormality associated with a monosomy 7. These patients showed a particularly poor prognosis, perhaps related to high EVI1 expression.22 The 5 other patients had a monosomy 7 associated with translocations, such as t(2;3), t(9;22), or with deletions del(5q), del(6q).
A few other groups have investigated the prognostic impact of MK status in several clinical conditions. An American study by Oran et al concerned 212 patients 18 to 68 years of age treated by allogeneic hematopoietic stem cell transplantation for AML, including 23 patients with MK.23 In multivariate analysis, MK was the only factor associated with shorter relapse-free survival in patients in first CR.23 In 3 other studies, including 68, 18, and 16 MK+ patients, respectively, the worse prognostic effect of MK was confirmed in patients younger than 60 years.24-26
Our results showed that none of the hypotheses tested in the clinical trials had modified the outcome and the poor prognosis of MK+ patients. None of the strategies has currently proved any efficacy on these MK+ unfavorable-risk AML, especially in elderly patients. The European Group for Blood and Marrow Transplantation conducted a study in a cohort of 278 patients with AML or myelodysplastic syndromes with chromosome 7 abnormalities, showing that the 63 MK+ patients did not benefit from allo-SCT, unlike MK− patients with chromosome 7 abnormalities.27
Up to now, there are no data supporting therapeutic recommendations for patients with MK+ AML. Considering the extremely poor prognosis of MK in older patients with a 7% 2-year OS as reported here, our opinion is to consider for these patients alternative treatment strategies. Rather than standard intensive treatment with a classic induction course, these patients should be offered, whenever possible, access to investigational drugs in the setting of prospective trials or less intensive, palliative chemotherapy combined with supportive care. AlloSCT might also be considered as an option for patients with the best Eastern Cooperative Oncology Group performance status.
In conclusion, this study of a cohort of elderly patients with cytogenetically unfavorable AML shows that MK, according to the criteria proposed by Breems et al,13 is also, as expected, an independent factor of very poor prognosis in older age. MK is more frequent in elderly patients and stands out as the major independent prognostic factor, distinguishing prognostic subgroups better than CK. It appears to be the most pertinent factor to stratify unfavorable cytogenetics in elderly AML patients and guide therapeutic decisions especially in future prospective trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all GOELAMS investigators for including patients and Roselyne Delepine and all clinical research assistants of the GOELAMS for ensuring the validity of clinical data.
Authorship
Contribution: A. Perrot performed the study, analyzed data, and wrote the manuscript; F.W. conceived and designed the study and wrote and reviewed the manuscript; I.L. reviewed cytogenetic data and reviewed the manuscript; M.C.B. wrote and reviewed the manuscript; P.G. reviewed statistical data; A. Pigneux, F.M., J.D., J.-L.H., C.B., J.-Y.C., C.H., C.R., N.V., B.L., M.O.-U., N.F., C.B., and E.R. included patients, reviewed data, and contributed to the manuscript; and N.I. gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of study group participants, see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Aurore Perrot, Service d'Hématologie et Médecine Interne, Centre Hospitalier Universitaire de Brabois, Rue du Morvan, 54500 Vandœuvre-lès-Nancy, France; e-mail: Aurore.Perrot@medecine.uhp-nancy.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal