Abstract
During vertebrate angiogenesis, Notch regulates the cell-fate decision between vascular tip cells versus stalk cells. Canonical Notch signaling depends on sequential proteolytic events, whereby interaction of Notch with membrane-anchored ligands triggers proteolytic processing, first by Adam10 and then presenilins. This liberates the Notch intracellular domain, allowing it to enter the nucleus and activate Notch-dependent genes. Here we report that conditional inactivation of Adam10 in endothelial cells (A10ΔEC) recapitulates the increased branching and density of the retinal vasculature that is also caused by interfering with Notch signaling. Moreover, A10ΔEC mice have additional vascular abnormalities, including aberrant subcapsular hepatic veins, enlarged glomeruli, intestinal polyps containing endothelial cell masses, abnormal endochondral ossification, leading to stunted long bone growth and increased pathologic neovascularization following oxygen-induced retinopathy. Our findings support a model in which Adam10 is a crucial regulator of endothelial cell-fate decisions, most likely because of its essential role in canonical Notch signaling.
Introduction
In the developing vasculature, appropriate vessel sprouting and branching is thought to be regulated by Notch signaling.1-10 Interference with Notch signaling, such as through postnatal loss of Notch-1 in endothelial cells, or hemizygosity of the Notch ligand Delta-like–4, or inhibition of γ-secretase, an enzyme that releases the Notch intracellular domain from its membrane anchor and allows it to activate Notch-dependent transcription in the nucleus, leads to increased branching and density in the developing retinal vasculature.11 Moreover, conditional inactivation of Notch-1 in endothelial cells leads to an arrest of prenatal developmental at embryonic day E9.5.5 Taken together, these findings point toward an essential role of Notch-1 in endothelial cells during early mouse development.
Canonical Notch signaling depends on sequential proteolytic events.12 The first is processing of Notch by furin in the secretory pathway.13 This generates the mature Notch heterodimer that can interact with a membrane-anchored Notch ligand on an adjacent cell. This, in turn, stimulates processing of Notch by Adam10, thereby generating a membrane anchored stub14,15 that is recognized by the presenilin complex.16,17 Intramembrane processing by presenilin releases the intracellular domain of Notch from its membrane anchor, allowing it to enter the nucleus and activate Notch-dependent gene transcription programs.12 Adam10 belongs to a family of membrane-anchored metalloproteinases containing “a disintegrin and metalloprotease” domains that serve to proteolytically process a variety of transmembrane proteins, including growth factors, cytokines, structural proteins, and receptors.18,19 Both Adam10 (Kuzbanian) and Adam17 (TACE) have been reported to process Notch-1 in cell-based assays, although the most recent reports clearly implicated Adam10 as the enzyme responsible for ligand-dependent processing of Notch.14,15,20 Moreover, studies in Drosophila,21 C elegans22,23 and mice24-27 have demonstrated that Adam10 is essential for Notch signaling in development. For example, mice lacking Adam10 die at E9.5 with defects resembling those observed in Notch-deficient mice,24 and lymphocyte-specific and neuronal-specific conditional inactivation of Adam10 in mice has recapitulated Notch dysfunction in those tissues, further implicating Adam10 as the functionally relevant Notch proteinase.25,26 The goal of this study was to generate conditional knockout mice lacking Adam10 in endothelial cells to evaluate the role of Adam10 in vascular development as well as in retinal angiogenesis and pathologic retinal neovascularization.
Methods
Reagents
All reagents were from Sigma-Aldrich, unless indicated otherwise. Rabbit anti–mouse Adam10 antibody (MAB946) was from R&D Systems, rat anti–mouse CD31 antibody (MEC 13.3) from BD Biosciences, rabbit anti–mouse Adam17 cytoplasmic domain antibody has been described previously,28 and rat anti–mouse MECA-32 monoclonal IgG2a antibody29 was from Developmental Studies Hybridoma Bank. Fluorescein conjugated Isolectin B4 was from Vector Labs and fluorescent mounting media from Dako. Sheep anti–rat conjugated magnetic beads (Dynabeads) were from Dynal Biotech ASA, and protease 3 was from Ventana Medical System.
Isolation of lung endothelial cells and Western blotting
Mouse strains, genotyping and breeding
To generate mice lacking Adam10 in endothelial cells, Adam10flox/flox mice25 were mated with Tie2-Cre transgenic mice31,32 (provided by Dr Tom Sato). Adam10flox/flox/Tie2-Cre mice (A10ΔEC) were maintained on a mixed genetic background (129P2/OlaHsd/C57BL6). Adam10flox/flox mice displayed no histopathologic abnormalities and were therefore used as controls for experiments with A10ΔE individuals. A previous study demonstrated that Tie2-Cre mice had no evident histopathologic defects.31 R26R reporter mice (The Jackson Laboratory, B6.129S4-Gt(ROSA)26Sortm1Sor/J) were used to monitor the expression of Cre-recombinase in Tie2-Cre individuals. Adam10flox/flox mice were mated to R26R individuals, and the offspring crossed to generate Adam10flox/flox, R26R/R26R mice. Adam10flox/flox, R26R/R26R mice, in turn, were mated to Adam10flox/flox, Tie2-Cre individuals and their offspring were used in X-gal staining analysis. All animal experiments were approved by the Internal Animal Care and Use Committee of the Hospital for Special Surgery.
Whole-mount Lac-Z Staining
A10ΔEC and A10ΔEC/R26R mice were euthanized and their eyes removed and fixed for 15 minutes in 4%PFA/0.5% glutaraldehyde. Then the retinas were isolated, washed 3 times in wash buffer (PBS, 2mM MgCl2, 5mM EGTA, 0.01% sodium deoxycholate, 0.02% Nonident P-40) and then stained for 3 hours at 37°C in staining buffer (wash buffer with 0.1% wt/vol bromo-chloro-indolyl-galactopyranoside, 5mM K3Fe(CN)6, and 5mM K4Fe(CN)6·6H2O). After staining, retinas were fixed in 4% PFA overnight and imaged on a Zeiss Axiovision SteREO Lumar.V12 Microscope.
Developmental Retinal Angiogenesis
Retinas were isolated from 5-day-old mice and incubated in blocking buffer (1% BSA, 100mM glycine, 0.5% vol/vol Triton-X 100 in PBS) for 1 hour at 4°C and then incubated in blocking buffer with 2μg/mL Isolectin B4 overnight at 4°C. A Zeiss AxioVert Observer.Z1 microscope was used in conjunction with a motorized stage to generate approximately 40 images at 200× magnification to capture the entire retina and Zeiss Axiovision V4.7 software was used to generate a contiguous retinal image. For quantification, lines bisecting each retinal “leaf” from the center of the optic disk to the angiogenic front were generated and measured, with 4 measurements made on each retina. These values were used to generate an average value of angiogenic front progression. To measure the coverage of vessels at the angiogenic front, rectangular fields (8 fields of 500μm × 200μm per retina for 17-21 retinas) were imported into Adobe Photoshop (see supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To quantify branching, loops generated by the developing vasculature were counted in these fields as shown in supplemental Figure 1B (red dots mark individual loops). To determine endothelial cell coverage, images were processed in Photoshop by setting a minimum signal threshold such that Isolectin B4 fluorescence registered at saturating levels (255) while background was set to minimum signal (0; see supplemental Figure 1C). This processing step was performed to standardize the variable background fluorescence present between retinal images and to aid in endothelial cell coverage calculation in ImageJ. After processing, ImageJ was used to determine the area of vessel coverage by measuring the integrated intensity of pixels across each image and image area, then applying the following formula: % coverage = ([integrated intensity])/[area × 255]) × 100. Eight fields from each retina were quantified. To determine tip cell density at the vascular front, tip cells (defined as endothelial cells possessing bursts of filopodia,11 marked by red dots in supplemental Figure 1D) were counted based on morphologic presence of filopodial collections (arrows) in 4 microscopic fields of 200μm × 200μm from each retina (15 retinas per group). Filopodia density at the vascular front was quantified by demarcating 100μm of vascular front (yellow line in supplemental Figure 1E) and counting filopodia protrusions11 (marked by red dots in supplemental Figure 1E) along that front (4 measurements per retina; 15 retinas per group). Statistical analysis was performed using a 2-tailed Student t test.
Oxygen Induced Retinopathy
Oxygen-induced retinopathy experiments were performed as described.33 Subsequently, animals were euthanized and their eyes removed, and the size of the central avascular area and the development of neovascular tuft was analyzed as described.31 A Wilcoxon-Mann-Whitney test was used to assess statistical signifigance.
Pathologic analysis
After euthanasia by CO2 (according to the guidelines of the American Veterinary Medical Association), all organs were examined grossly and fixed in 10% neutral buffered formalin or 4% paraformaldehyde. Tissues were processed routinely for histology and embedded in paraffin, sectioned at 4 microns thickness stained with H&E and examined. Selected tissues were stained with the Masson trichrome. The following tissues were examined: diaphragm, semimembranosus and semitendinosus muscles, sciatic nerve, heart, thymus, lungs, kidneys, salivary glands, lymph nodes (submandibular, mesenteric), stomach, duodenum, jejunum, ileum, cecum, colon, pancreas, thyroid gland, esophagus, trachea, adrenal glands, liver, gallbladder, spleen, testes, epididymides, seminal vesicles, prostate gland, urinary bladder, skin (trunk, head, digits), femur, tibia (with stifle joint), humerus (with shoulder joint), tarsus, metatarsus and digit, sternum (including bone marrow), brain, pituitary gland, ears, eyes, nose, mouth, teeth, vertebrae with discs, spinal cord. Smears of femoral bone marrow were prepared, stained with Wright-Giemsa and examined.
Serum chemistry
For serum chemistry, blood was collected in tubes containing a serum separator, centrifuged, and the serum was analyzed by an Olympus AU400 analyzer. The following parameters were measured: alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ glutamyltransferase (GGT), albumin, total protein, globulin, total bilirubin, blood urea nitrogen (BUN), creatinine, cholesterol, glucose, calcium, phosphorus, total carbon dioxide (TCO2), chloride, potassium, sodium, albumin/globulin (A/G) ratio, BUN/creatinine (B/C) ratio, sodium/potassium (Na/K) ratio, osmolality (calculated), and anion gap.
Hematology
For hematology, blood was collected in tubes containing EDTA, and analyzed by a Sysmex XTV analyzer. Blood smears were prepared, stained with Wright-Giemsa, and examined. The following parameters were measured: total white blood cell count, differential and absolute counts for neutrophils, lymphocytes, monocytes, eosinophils, and basophils, hematocrit, red blood cell count, hemoglobin, mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and platelet count. Manual relative reticulocyte counts were performed on new methylene blue-stained blood smears, and absolute counts were calculated.
Analysis of embryos at different stages of development
After timed matings, embryos were isolated at the indicated time points by dissecting the uterine horns in ice-cold PBS. For CD31 staining, embryos were fixed in 4% PFA overnight and dehydrated with an increasing series of methanol. Six percent H2O2 in methanol was used to saturate endogenous peroxidase activity, followed by rehydration with a decreasing series of methanol in PBS. After blocking for 2 hours in normal goat serum, 5μg/mL of rat anti–mouse CD31 was added overnight and subsequently washed for 6 hours in PBS. Biotinylated goat anti–rat IgG antibody was added to embryos overnight and washed for 6 hours in PBS. Finally, embryos were incubated with streptavidin-HRP overnight followed by thorough washing with PBS. Embryos were developed using DAB according to the manufacturer's instructions (Vector). For X-gal staining, embryos were fixed in 2% PFA, 0.5% glutaraldehyde for 1 hour at 4°C. Embryos were washed in wash buffer (2mM MgCl2, 5mM EGTA, 0.01% sodium deoxycholate, 0.02% Nonident P-40) 3 times for 10 minutes, and were incubated for ∼ 5 hours in staining solution (washing solution with 50μg/mL X-gal, 5mM K3Fe(CN)6, and 5mM K4Fe(CN)6). After staining, embryos were post-fixed in 4% PFA overnight. Immunhistochemical detection of bound MECA-32 antibodies (used at 3 μg/mL) was carried out at the Molecular Cytology Core Facility of MSKCC, using a protocol established in the Core Facility, with a Discovery XT processor (Ventana Medical Systems). Briefly, the samples were blocked for 30 minutes with 10% normal rabbit serum, 2% BSA, then Protease 3 was added for antigen retrieval for 4 minutes. The incubation with the primary antibody was for 7 hours, followed by 60 minutes incubation with biotinylated rabbit anti–rat IgG (Vector, BA-4000, in 1:200 dilution), Blocker D, Streptavidin-HRP and DAB detection kit (Ventana Medical Systems) following the manufacturers instructions.
Results
Generation of mice lacking Adam10 in endothelial cells
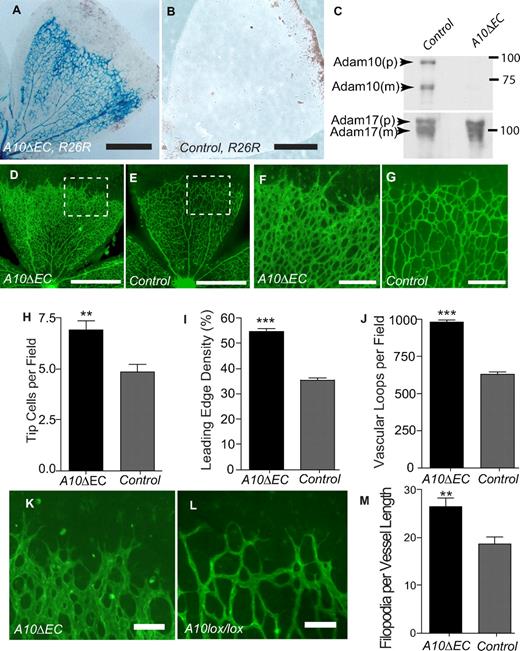
Mice carrying floxed alleles of Adam1025 were crossed with Tie2-Cre mice31,32 to generate animals lacking Adam10 in endothelial cells (Adam10flox/flox/Tie2-Cre, referred to as A10ΔEC). A10ΔEC mice were born at the expected Mendelian ratio from crosses of A10ΔEC mice with Adam10flox/flox controls (n = 123; A10ΔEC: 64 [52%], controls: 59 [48%]) and were viable and fertile. However, an increased lethality of A10ΔEC mice became evident starting at 10 weeks of age, with only ∼ 25% of A10ΔEC still surviving at 50 weeks, compared with 100% of controls (supplemental Figure 2). The endothelial-specific expression of Tie2-Cre was corroborated using a Rosa-26–reporter (R26R) that drives expression of Lac-Z after Cre-mediated recombination. X-gal staining of whole-mounted retinas from A10ΔEC/R26R mice highlighted the retinal vascular tree in blue, demonstrating effective recombination of LoxP sites in endothelial cells by Tie2-Cre (Figure 1A) whereas no staining was seen in retinas from Adam10flox/flox/R26R mice lacking Tie2-Cre (Figure 1B). Western blot analysis of endothelial cells isolated from lungs of A10ΔEC animals showed a strong reduction of Adam10 compared with controls, with comparable levels of Adam17 (Figure 1C). To corroborate that the Cre-mediated deletion of the floxed alleles of Adam10 inactivates Adam10, we crossed Adam10wt/flox mice with animals expressing Cre under control of the ubiquitously expressed EIIa-promoter to delete Adam10 in the germ line. Matings of the resulting Adam10+/− individuals produced Adam10−/− embryos that died at E9.5, and thus resembled the previously reported Adam10−/− mice24 (supplemental Figure 3A,B).
Adam10 deficiency in endothelial cells causes increased vascular density at the front of the developing retinal vasculature. (A,B) Lac-Z staining of retinas from A10ΔEC-R26R or A10flox/flox-R26R control mice. (C) Immunoblot of Adam10 and Adam17 in primary endothelial cells isolated from lungs of A10flox/flox control or A10ΔEC mice (p: pro-form, m: mature form). (D-G) Isolectin-B4 staining of retinas from 5-day-old A10ΔEC or control mice. Panels F and G correspond to the area marked by insets in panels D and E, respectively. (H-J) Quantitative analysis of tip cell density (H, A10ΔEC: 6.9 ± 1.8 per 100 μm of vascular front, n = 15; controls: 4.9 ± 1.4, n = 15), endothelial cell coverage at the front of the retinal vascular tree (I, A10ΔEC: 54.8% ± 4.42, n = 17; controls: 35.3% ± 3.8, n = 21), and vascular loops per field (J, A10ΔEC: 980 loops per mm2 ± 65, n = 17, controls 628 ± 68 per mm2, n = 21). (K,L) Micrographs of tip cells at the leading edge of the developing retinal vascular tree in A10ΔEC and control mice, and quantitative analysis of filopodia density (M, A10ΔEC: 26.4 ± 6.8 per 100 μm vessel length, n = 15, controls: 18.6 ± 5.6, n = 15). Please see “Evaluation of developmental retinal angiogenesis” and supplemental Figure 1 for details. Error bars represent mean ± SEM. ** indicates P < .01, and *** indicates P < .001 in a 2-tailed Student t test. Scale bars in panels A-B: 300 μm; D-E: 400 μm; F-G: 100 μm, K-L: 20 μm.
Adam10 deficiency in endothelial cells causes increased vascular density at the front of the developing retinal vasculature. (A,B) Lac-Z staining of retinas from A10ΔEC-R26R or A10flox/flox-R26R control mice. (C) Immunoblot of Adam10 and Adam17 in primary endothelial cells isolated from lungs of A10flox/flox control or A10ΔEC mice (p: pro-form, m: mature form). (D-G) Isolectin-B4 staining of retinas from 5-day-old A10ΔEC or control mice. Panels F and G correspond to the area marked by insets in panels D and E, respectively. (H-J) Quantitative analysis of tip cell density (H, A10ΔEC: 6.9 ± 1.8 per 100 μm of vascular front, n = 15; controls: 4.9 ± 1.4, n = 15), endothelial cell coverage at the front of the retinal vascular tree (I, A10ΔEC: 54.8% ± 4.42, n = 17; controls: 35.3% ± 3.8, n = 21), and vascular loops per field (J, A10ΔEC: 980 loops per mm2 ± 65, n = 17, controls 628 ± 68 per mm2, n = 21). (K,L) Micrographs of tip cells at the leading edge of the developing retinal vascular tree in A10ΔEC and control mice, and quantitative analysis of filopodia density (M, A10ΔEC: 26.4 ± 6.8 per 100 μm vessel length, n = 15, controls: 18.6 ± 5.6, n = 15). Please see “Evaluation of developmental retinal angiogenesis” and supplemental Figure 1 for details. Error bars represent mean ± SEM. ** indicates P < .01, and *** indicates P < .001 in a 2-tailed Student t test. Scale bars in panels A-B: 300 μm; D-E: 400 μm; F-G: 100 μm, K-L: 20 μm.
Evaluation of developmental retinal angiogenesis
Notch signaling serves as a negative regulator of vessel branching during developmental retinal angiogenesis.3,4,6-9,11 When vascular branching was assessed on whole-mounted FITC-isolectin B4-stained retinas from 5-day-old A10ΔEC mice, a higher vascular density with increased numbers of branching tip cells and increased vascular loops was observed in the leading third of the developing vascular tree compared with controls (Figure 1D-L). In addition, A10ΔEC tip cells had a greater number of filopodia than controls (Figure 1K-M). The distance between the leading edge of the retinal vascular tree and its origin at the optic nerve was comparable in A10ΔEC mice and littermate controls (1.05mm2 ± 0.09mm2, n = 21 and 1.02mm2 ± 0.07mm2, n = 17) so there was no significant difference in the growth of the retinal vasculature. A similar rise in vascular density was seen in the deep retinal vascular plexus in 12-day old mice, while it had largely resolved in superficial vascular beds (supplemental Figure 4A-D). The retinal vasculature in adult A10ΔEC mice appeared normal (supplemental Figure 4G-J), suggesting that the abnormalities during vascular development are transient and later remodel.
Oxygen-induced retinopathy
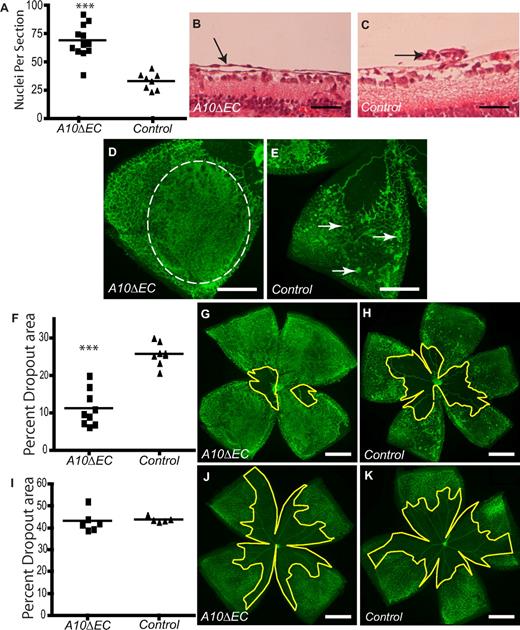
To investigate the role of Adam10 in pathologic retinal neovascularization, A10ΔEC mice and littermate controls were subjected to oxygen-induced retinopathy (OIR), a mouse model for proliferative retinopathies.33,34 In this model, 7-day-old mice are placed in a chamber with 75% oxygen and then returned to room air 5 days later (p12). The resulting relative hypoxia triggers a neovascular response that leads to growth of vessels into the central avascular area and also to the development of pathologic neovascular tufts at p17. Retinas from A10ΔEC mice had a significantly increased number of endothelial cells that had crossed the internal limiting membrane compared with controls (Figure 2A-C). In a whole-mount analysis, these resembled thin layers of endothelial cells instead of the typical compact neovascular tufts observed in controls (Figure 2D,E). Moreover, the revascularization of the central avascular area at p17 was increased in A10ΔEC mice (Figure 2F-H), even though the size of the avascular area at the end of oxygen treatment at p12 was comparable to controls (Figure 2I-K), suggesting that Adam10 serves to limit both the revascularization of the avascular area as well as the extent of pathologic neovascularization.
Oxygen Induced Retinopathy (OIR) in A10ΔEC mice. (A) Quantitative analysis of cell nuclei found vitreal to the internal limiting membrane after OIR in A10ΔEC and control mice (A10ΔEC: 68.9 ± 4.2, n = 12; controls: 33.3 ± 2.6, n = 8). (B,C) Histologic analysis revealed an abnormal sheet-like vascular structure (marked by an arrow) in an A10ΔEC retina (B) and a typical neovascular tuft (pointed by an arrow) in a control retina (C). (D-E) Micrographs of a segment of an Isolectin B4-stained retina from an A10ΔEC mouse showed a sheet-like vascular structure (outlined by a dotted line in panel D), whereas typical neovascular tufts were visible in a retina from a control mouse (marked by arrows in panel E). (F) Quantification of the size of the central avascular area in A10ΔEC eyes compared with controls after a 5-day period at room air (p17; A10ΔEC: 11.3 ± 1.5%, n = 9; controls: 25.7% ± 1.1% n = 8), and corresponding representative micrographs of A10ΔEC (G) or control (H) retinas. (I) Quantification of the avascular area in A10ΔEC (J) and control (K) retinas after 5 days at 75% oxygen (p12; A10ΔEC: 43.2 ± 2.0%, n = 6; controls: 43.8% ± 0.6%, n = 5). The central avascular area is bounded by a yellow line in panels G,H,J and K. ***P < .001 in a 2-tailed Student t test. Scale bars in panels B-C: 50 μm; D-E: 400 μm; G-H,J-K: 750 μm.
Oxygen Induced Retinopathy (OIR) in A10ΔEC mice. (A) Quantitative analysis of cell nuclei found vitreal to the internal limiting membrane after OIR in A10ΔEC and control mice (A10ΔEC: 68.9 ± 4.2, n = 12; controls: 33.3 ± 2.6, n = 8). (B,C) Histologic analysis revealed an abnormal sheet-like vascular structure (marked by an arrow) in an A10ΔEC retina (B) and a typical neovascular tuft (pointed by an arrow) in a control retina (C). (D-E) Micrographs of a segment of an Isolectin B4-stained retina from an A10ΔEC mouse showed a sheet-like vascular structure (outlined by a dotted line in panel D), whereas typical neovascular tufts were visible in a retina from a control mouse (marked by arrows in panel E). (F) Quantification of the size of the central avascular area in A10ΔEC eyes compared with controls after a 5-day period at room air (p17; A10ΔEC: 11.3 ± 1.5%, n = 9; controls: 25.7% ± 1.1% n = 8), and corresponding representative micrographs of A10ΔEC (G) or control (H) retinas. (I) Quantification of the avascular area in A10ΔEC (J) and control (K) retinas after 5 days at 75% oxygen (p12; A10ΔEC: 43.2 ± 2.0%, n = 6; controls: 43.8% ± 0.6%, n = 5). The central avascular area is bounded by a yellow line in panels G,H,J and K. ***P < .001 in a 2-tailed Student t test. Scale bars in panels B-C: 50 μm; D-E: 400 μm; G-H,J-K: 750 μm.
Vascular development at E9.5
The development of the retinal vasculature is considered to be a model system for embryonic vascular development. However, the vascular tree in the brain, cerebellum and somites of A10ΔEC embryos at E9.5 stained with the endothelial cell marker CD31 was indistinguishable from that of littermate controls. Figure 3A-D shows representative examples of a total of 8 embryos (A10ΔEC: 4; controls: 4 analyzed in a blinded manner). Moreover, the distribution of vessels in hindbrain sections of E9.5 embryos was comparable in A10ΔEC embryos and littermate controls (Figures 3E-F; A410ΔEC: 8.7 per 400 μm ± 0.9, n = 9 sections and controls: 8.3 per 400 μm ± 1.0, n = 9 sections). Staining of E8.5 embryos carrying Tie2-Cre and the R26R reporter with X-gal demonstrated an irregular and patchy expression of Tie2-Cre (Figure 3G), suggesting that the lack of an evident branching defect in the developing vasculature of E9.5 A10ΔEC embryos could be explained by the incomplete expression of the Tie2-Cre transgene in endothelial cells at this stage.
Analysis of developmental angiogenesis at E9.5. (A-D) Micrographs of CD31-stained whole-mounted A10ΔEC (A,C) and control (B,D) embryos at E9.5 (superficial cranial vessels are marked by arrows, and intersomitic vessels by arrowheads). (E,F) Representative micrographs of CD31-stained sections of the vasculature of the fourth ventricular floor in A10ΔEC (E) and control (F) embryos at E9.5 showed a similar distribution of small caliber vessels (arrowheads). (G) Whole-mounted LacZ staining of a Tie2-Cre/R26R control E8.5 embryo showed patchy β-galactosidase staining within cranial vessels (arrow). Scale bars in panels A-B,G: 600 μm; C-D: 400 μm; E-F: 30 μm.
Analysis of developmental angiogenesis at E9.5. (A-D) Micrographs of CD31-stained whole-mounted A10ΔEC (A,C) and control (B,D) embryos at E9.5 (superficial cranial vessels are marked by arrows, and intersomitic vessels by arrowheads). (E,F) Representative micrographs of CD31-stained sections of the vasculature of the fourth ventricular floor in A10ΔEC (E) and control (F) embryos at E9.5 showed a similar distribution of small caliber vessels (arrowheads). (G) Whole-mounted LacZ staining of a Tie2-Cre/R26R control E8.5 embryo showed patchy β-galactosidase staining within cranial vessels (arrow). Scale bars in panels A-B,G: 600 μm; C-D: 400 μm; E-F: 30 μm.
Analysis of the vasculature in liver, heart and diaphragm
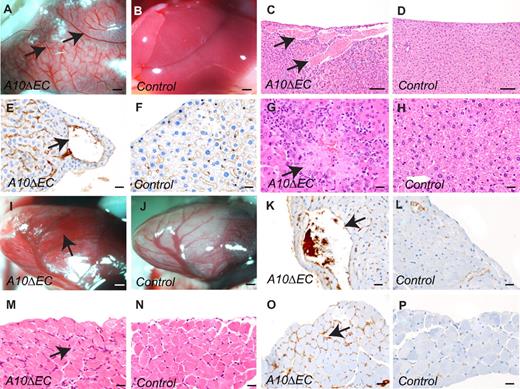
Histopathologic evaluation of 6-week-old A10ΔEC animals uncovered a variety of vascular abnormalities (n = 8, A10ΔEC: 4; controls: 4). The most evident defect on macroscopic examination of the abdominal cavity was the presence of large-caliber vessels on the liver surface and marked pallor of the liver that were not visible in control animals (Figure 4A-B). The enlarged vessels communicated with hepatic sinusoids, which were often moderately dilated (Figure 4C-D). The abnormal vessels were thin-walled, lined by a single layer of MECA-32–positive endothelial cells,29 and without a visible tunica media, suggesting that they are veins or venules (Figures 4E-F). Moreover, there were randomly distributed foci of hepatic coagulative necrosis in the adjacent parenchyma, which correlated with elevation of liver enzymes in all A10ΔEC individuals (n = 4) compared with controls (n = 4) (alanine transaminase, 2-10 fold elevation, aspartate aminotransferase, 1.5-9 fold elevation; Figures 4G-H and data not shown). Similar thin-walled vessels lined by MECA-32–positive cells were seen in the subepicardial myocardium (Figure 4I-L). In the diaphragm, there was diffuse endomysial hypercellularity, characterized by MECA-32–positive cells forming smaller, capillary-like vascular structures (Figure 4M-P). These abnormalities were not visible in control animals and had not yet developed in A10ΔEC embryos at E15.5, where the distribution of MECA-32–stained cells in sections of the liver, diaphragm or heart were comparable with controls (supplemental Figure 5A-D).
Histopathologic analysis of the liver, heart and diaphragm in adult mice. (A-D) Livers from A10ΔEC animals had enlarged vessels under the surface in gross (A) and histologic (C) specimens that were not present in livers from control animals (B,D). (E-F) MECA-32 immunohistochemistry of liver sections from A10ΔEC (E) and control (F) individuals showed positive staining for endothelial cells lining vascular structures. Arrows mark the abnormally enlarged vessels in panels A,C and E. (G,H) Liver sections from A10ΔEC mice demonstrated areas of coagulative necrosis (G, arrow) not present in controls (H). (I,J) A heart from an A10ΔEC animal showed numerous, dilated, superficial blood-filled vascular structures (marked by an arrow in panel I) that were not present in a heart from a control animal (J). (K,L) MECA-32 immunohistochemistry of a heart section from an A10ΔEC mouse (K) showed positive staining for endothelial cells surrounding the enlarged vascular structures (pointed by an arrow in panel K) that were not present in a control heart (I). (M-N) Histopathologic analyses of muscular diaphragm specimens revealed increased cellularity between myofibers in A10ΔEC animals (M, arrow) compared with controls (N). (O-P) MECA-32 immunohistochemistry of diaphragm sections from A10ΔEC (O) and control (P) individuals showed positive staining of cells present between myofibers of A10ΔEC diaphragms (arrow in panel O). The micrographs of vascular abnormalities on H&E-stained sections are representative of all animals analyzed for each genotype (n = 6). Scale bars in panels A-B: 1000 μm; C-D: 100 μm; E-H,K-P: 20 μm; I-J: 500 μm.
Histopathologic analysis of the liver, heart and diaphragm in adult mice. (A-D) Livers from A10ΔEC animals had enlarged vessels under the surface in gross (A) and histologic (C) specimens that were not present in livers from control animals (B,D). (E-F) MECA-32 immunohistochemistry of liver sections from A10ΔEC (E) and control (F) individuals showed positive staining for endothelial cells lining vascular structures. Arrows mark the abnormally enlarged vessels in panels A,C and E. (G,H) Liver sections from A10ΔEC mice demonstrated areas of coagulative necrosis (G, arrow) not present in controls (H). (I,J) A heart from an A10ΔEC animal showed numerous, dilated, superficial blood-filled vascular structures (marked by an arrow in panel I) that were not present in a heart from a control animal (J). (K,L) MECA-32 immunohistochemistry of a heart section from an A10ΔEC mouse (K) showed positive staining for endothelial cells surrounding the enlarged vascular structures (pointed by an arrow in panel K) that were not present in a control heart (I). (M-N) Histopathologic analyses of muscular diaphragm specimens revealed increased cellularity between myofibers in A10ΔEC animals (M, arrow) compared with controls (N). (O-P) MECA-32 immunohistochemistry of diaphragm sections from A10ΔEC (O) and control (P) individuals showed positive staining of cells present between myofibers of A10ΔEC diaphragms (arrow in panel O). The micrographs of vascular abnormalities on H&E-stained sections are representative of all animals analyzed for each genotype (n = 6). Scale bars in panels A-B: 1000 μm; C-D: 100 μm; E-H,K-P: 20 μm; I-J: 500 μm.
Intestinal polyps and enlarged glomeruli in A10ΔEC mice
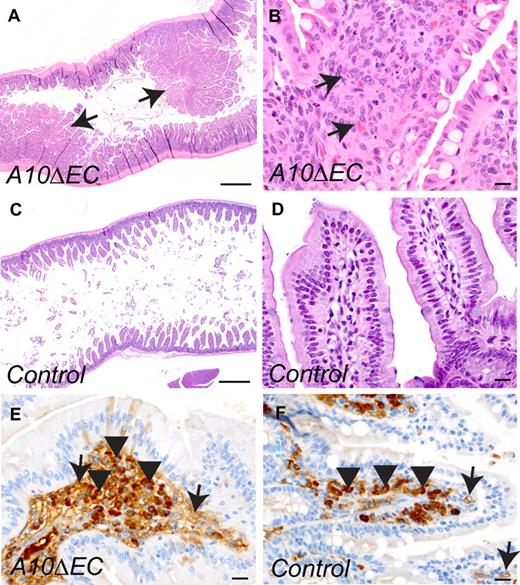
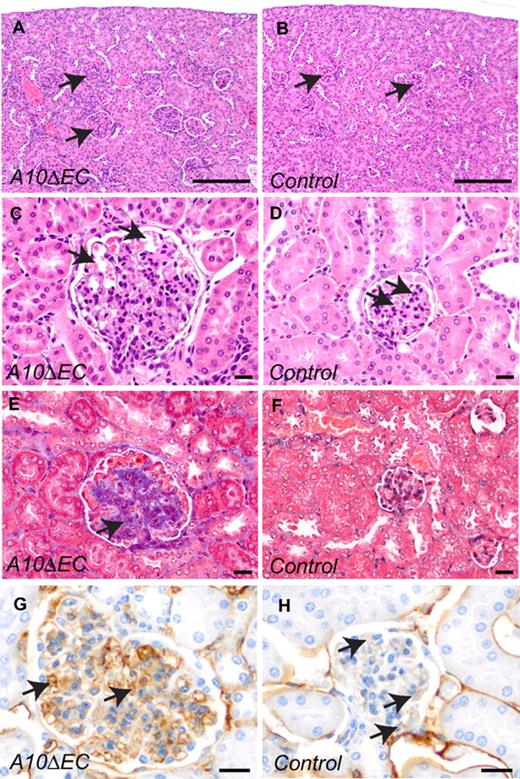
Analysis of the small intestine of A10ΔEC mice revealed numerous hyperplastic sessile or pedunculated mucosal polyps containing multifocal clusters of atypical cells in the lamina propria (Figure 5A-B), which were not observed in control mice (Figure 5C-D). The atypical cellular masses in the polyps of A10ΔEC mice were composed of densely arranged plump MECA-32–positive spindle cells, with scattered single cell necrosis, and small clefts containing red blood cells, suggesting formation of poorly differentiated vascular structures (Figure 5B,E). By comparison, only a fraction of the cells within the lamina propria of control mice stained with MECA-32 (Figure 5F, please note strong background staining of plasma cells, arrow heads). Moreover, kidney sections from A10ΔEC mice revealed enlarged and hypercellular glomeruli with occasionally dilated peripheral capillaries compared with controls (Figure 6A-D) and an increased amount of mesangial collagenous matrix, as detected by H&E and Masson's trichrome staining (Figure 6E-F). There was stronger MECA-32 staining in glomeruli of adult A10ΔEC mice than in controls, consistent with an increase in endothelial cells (Figure 6G-H). At embryonic stage E14.5, the MECA-32 staining in the developing intestine and kidney where similar in A10ΔEC mice and littermate controls (supplemental Figure 5E-H), suggesting that the expansion of MECA-32–positive cells in the intestinal polyps and enlarged glomeruli occurs at a later stage.
A10ΔEC mice develop intestinal polyps. (A-D) H&E staining of intestine from an A10ΔEC (A-B) and control (C-D) mouse revealed hyperplastic intestinal polyps (indicated by arrows in panel A) with marked hypercellularity within the lamina propria of A10ΔEC individuals (marked by arrows in panel B). (E-F) Almost all cells within the lamina propria of the enlarged polyps in A10ΔEC intestine stained positively for MECA-32 (E, arrow), while less than half the cells were MECA-32–positive in the corresponding area in control villi (F). Please note that the round dark-staining cells marked by arrowheads most likely represented plasma cells and not endothelial cells. Scale bars in panels A,C: 500 μm; B,D-F: 20 μm.
A10ΔEC mice develop intestinal polyps. (A-D) H&E staining of intestine from an A10ΔEC (A-B) and control (C-D) mouse revealed hyperplastic intestinal polyps (indicated by arrows in panel A) with marked hypercellularity within the lamina propria of A10ΔEC individuals (marked by arrows in panel B). (E-F) Almost all cells within the lamina propria of the enlarged polyps in A10ΔEC intestine stained positively for MECA-32 (E, arrow), while less than half the cells were MECA-32–positive in the corresponding area in control villi (F). Please note that the round dark-staining cells marked by arrowheads most likely represented plasma cells and not endothelial cells. Scale bars in panels A,C: 500 μm; B,D-F: 20 μm.
A10ΔEC mice display marked glomerular pathology. (A-D) H&E staining of kidney sections revealed enlarged glomeruli (A) with increased cellularity (C) in A10ΔEC mice (A,C) compared with control mice (B,D; arrows point to glomeruli in panels A and B, and to glomerular microvessels in panels C and D). (E-F) Masson trichrome staining revealed increased amounts of blue-staining material consistent with increased collagen deposition within glomeruli of A10ΔEC individuals (indicated by arrow in panel E) not present in control individuals (F). MECA-32 immunohistochemistry of kidney sections showed positive staining of most cells present within glomeruli of an A10ΔEC mouse (H, arrows), with only few capillaries stained in a glomerulus of a control mouse (G, arrows). Scale bars in panels A-B: 200 μm; C-H: 20 μm.
A10ΔEC mice display marked glomerular pathology. (A-D) H&E staining of kidney sections revealed enlarged glomeruli (A) with increased cellularity (C) in A10ΔEC mice (A,C) compared with control mice (B,D; arrows point to glomeruli in panels A and B, and to glomerular microvessels in panels C and D). (E-F) Masson trichrome staining revealed increased amounts of blue-staining material consistent with increased collagen deposition within glomeruli of A10ΔEC individuals (indicated by arrow in panel E) not present in control individuals (F). MECA-32 immunohistochemistry of kidney sections showed positive staining of most cells present within glomeruli of an A10ΔEC mouse (H, arrows), with only few capillaries stained in a glomerulus of a control mouse (G, arrows). Scale bars in panels A-B: 200 μm; C-H: 20 μm.
Defects in long bone growth and increased erythropoiesis
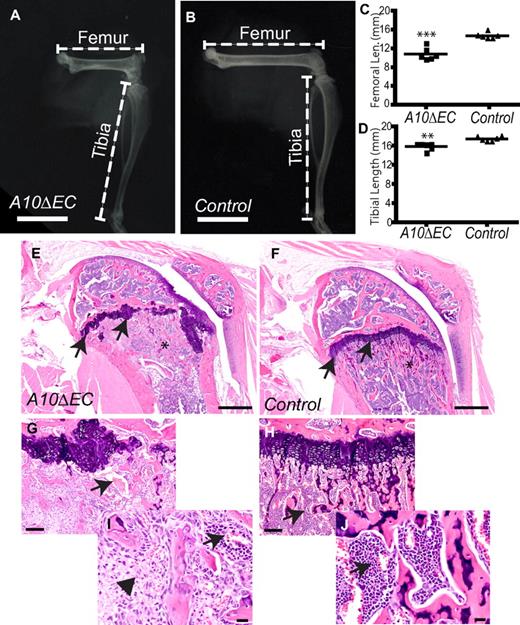
Live adult A10ΔEC mice could be visually distinguished from their littermate controls because of their shorter hind limbs, caused by significantly shortened femurs (Figure 7A-C). The tibiae and humeri were also slightly shorter in A10ΔEC mice than in controls (Figures 7A-B,D and data not shown), whereas the length of the vertebral column and the size and appearance of other skeletal structures such as tarsals, metatarsals and ribs were not detectably affected (supplemental Figure 6 and data not shown). Sections of the distal femoral growth plate of A10ΔEC mice uncovered abnormalities such as discontinuous and poorly organized zones of proliferating and hypertrophic chondrocytes in which the overall thickness of these zones varied substantially (Figure 7E-H). The primary spongiosa showed areas of increased and decreased bone density, as well as multifocal replacement of hematopoietic cells in intertrabecular spaces by loosely arranged atypical spindle cells (Figure 7I-J). Finally, histologic analysis of the spleen revealed marked expansion of the red pulp by increased numbers of erythroid precursors in A10ΔEC mice (supplemental Figure 7), and cytologic evaluation of the bone marrow showed expansion of the immature and mature erythroid pools, with normal cellular morphology and maturation sequence (supplemental Table 1 and data not shown). The hemoglobin, hematocrit, and red blood cell counts were lower in A10ΔEC mice compared with controls, and relative reticulocyte counts were elevated (supplemental Figure 8), raising the possibility of hemolysis accompanied by a regenerative erythroid response, perhaps caused by flow through the disorganized vascular beds of mutant glomeruli and intestinal polyps.
Long bone growth is impeded in A10ΔEC mice. (A,B) Radiographs of the hind limb of an A10ΔEC mouse (A) revealed a substantially shorter femur than in a control mouse (B). (C-D) Quantification showed significant reduction in the length of the femur and tibia in A10ΔEC mice compared with controls (C; femur length, A10ΔEC:10.9 mm ± 0.5 n = 6; controls: 14.7 mm ± 0.3 n = 6; D; tibia length, A10ΔEC: 15.9 mm ± 0.3 n = 6; controls: 17.4 mm ± 0.2 n = 6,). (E-J) H&E staining of femoral condyles from A10ΔEC (E,G,I) or control mice (F,H,J) revealed an abnormal growth plate (arrows in panel E), disturbed trabecular bone architecture (asterisk in E), abnormally oriented and enlarged vessels (arrow in panel G) and aberrant spindle shaped stromal cells (arrowhead in panel I) in A10ΔEC specimens compared with the normal growth plate (arrows in panel F), trabecular bone (asterisk in panel F), vessels (arrow in panel H) and compact hematopoietic cells within the marrow compartment (arrows in I,J) in controls. Data show mean ± SEM. Scale bars in panels A-B: 8 mm; E-F: 500 μm; G-H: 100 μm; I-J: 20 μm.
Long bone growth is impeded in A10ΔEC mice. (A,B) Radiographs of the hind limb of an A10ΔEC mouse (A) revealed a substantially shorter femur than in a control mouse (B). (C-D) Quantification showed significant reduction in the length of the femur and tibia in A10ΔEC mice compared with controls (C; femur length, A10ΔEC:10.9 mm ± 0.5 n = 6; controls: 14.7 mm ± 0.3 n = 6; D; tibia length, A10ΔEC: 15.9 mm ± 0.3 n = 6; controls: 17.4 mm ± 0.2 n = 6,). (E-J) H&E staining of femoral condyles from A10ΔEC (E,G,I) or control mice (F,H,J) revealed an abnormal growth plate (arrows in panel E), disturbed trabecular bone architecture (asterisk in E), abnormally oriented and enlarged vessels (arrow in panel G) and aberrant spindle shaped stromal cells (arrowhead in panel I) in A10ΔEC specimens compared with the normal growth plate (arrows in panel F), trabecular bone (asterisk in panel F), vessels (arrow in panel H) and compact hematopoietic cells within the marrow compartment (arrows in I,J) in controls. Data show mean ± SEM. Scale bars in panels A-B: 8 mm; E-F: 500 μm; G-H: 100 μm; I-J: 20 μm.
Discussion
The principal goal of this study was to elucidate the function of the metalloproteinase Adam10 in endothelial cells during mouse development and adult homeostasis. Inactivation of Adam10 in endothelial cells resulted in severe vascular abnormalities in the developing mouse retina and in various specialized vascular compartments. The phenotype of the developing retinal vascular tree in A10ΔEC mice at postnatal day P5 had a strong resemblance with that described for mice that are heterozygous for the endothelial Notch-ligand Dll4 or after a conditional temporal inactivation of Notch1, or after treatment with γ-secretase inhibitors.11 However, mice lacking Notch1 in endothelial cells die at E9.5 with severe defects in cardiovascular development,5 whereas the A10ΔEC mice described here are viable and fertile, despite their vascular abnormalities in the liver, heart, kidney and intestine. Interestingly, a recently reported conditional deletion of Adam10 using a different Tie2-Cre driver line resulted in early embryonic lethality.35 Because it is now well established that some Tie2-Cre transgenes can induce germ line deletion of floxed alleles,36 one possible cause for the difference in phenotypes could be a more ubiquitous expression of the Tie2-Cre driver used by Zhang et al, although a contribution of different genetic backgrounds cannot be ruled out. Interestingly, the activation of an R26R Lac-Z reporter at embryonic day E8.5 by the Tie2-Cre used in our study had a mosaic pattern, raising the possibility that a later onset of expression of this Tie2-Cre, perhaps coupled with a lack of germ line expression could provide an explanation for the survival of the A10ΔEC mice used here. We excluded that recombination of the floxed Adam10 allele we used could lead to a hypomorphic phenotype by generating Adam10−/− mice after germ line deletion of floxed Adam10. These animals displayed severe defects in somitogenesis and heart development at E9.5, and therefore resembled previously described Adam10−/− knockout mice.24 The viability of the A10ΔEC mice generated in this study thus provided a unique opportunity to study how deleting Adam10 in endothelial cells affects vascular structures in developing and adult mice.
The large vessels under the liver capsule of adult A10ΔEC mice are consistent with a defect in Notch signaling since they resemble those described in transgenic mice overexpressing the Notch1 extracellular domain,37 which is likely to interfere with Notch signaling in a dominant negative manner. However, unlike the abnormal vessels throughout the liver that were reported by Li et al, the enlarged vessels in A10ΔEC mice were confined to the subcapsular region, and were not seen deeper within the parenchyma. In addition, we observed an increased vascularity in the muscular diaphragm and epicardium, which, together with the liver capsule, have developmental origins from the septum transversum mesenchyme.38 The enlarged vascular structures in all 3 tissues were thin-walled, surrounded by a single layer of endothelial cell without a tunica media, suggesting that they are small veins or venules, and therefore that Adam10 has a role in limiting the size of these veins during development, as has been previously demonstrated for Notch.6,8 Interestingly, there were no evident defects in the brain, lung and appendicular muscle of A10ΔEC mice (data not shown), suggesting that these tissues and their vascular beds develop normally in the absence of Adam10. However, we cannot rule out temporary defects that are later remodeled, as in the retinal vasculature, where the transient nature of the increased branching phenotype can most likely be attributed to vascular remodeling and pruning by leukocytes, which also occurs during normal retinal angiogenesis.39
Additional vascular defects were uncovered in renal glomeruli and in the small intestine of adult A10ΔEC mice. Mutant glomeruli were markedly enlarged and hypercellular, with increased staining for the endothelial cell marker MECA-32,29 suggesting that this morphologic abnormality resulted from an expansion of endothelial cells. Likewise, the numerous intestinal polyps in A10ΔEC mice contained masses of cells expressing MECA-32, which appeared to have caused these polyps by expanding within the underlying lamina propria. Because endothelial cells in glomeruli and intestinal villi each represent vascular niches40 with specialized functions (filtration or absorption, respectively), we hypothesize that Adam10 has a role in regulating cell fate decisions in these specialized vascular cells, leading to their abnormal expansion in the absence of Adam10. Future studies will be required to better understand the developmental origin and properties of these cells, and to determine whether there is a functional equivalent of tip and stalk cells in the development of the vasculature in glomeruli and intestinal villi.
The significantly shortened femurs in A10ΔEC mice are presumably also caused by abnormalities in the specialized vasculature invading the growth plate, as previous studies have uncovered important roles for angiogenesis during bone growth by endochondral ossification.41-44 Interestingly, the growth of other long bones was less strongly or not affected in A10ΔEC mice, even though sections of all long bones analyzed exhibited abnormal morphology of the growth plate. Perhaps the growth of the femur is more strongly affected than that of other long bones in A10ΔEC mice because it has the largest diameter of all long bones, which could conceivably further exacerbate the hypoxia gradient that develops toward the center of growing long bones.44 A more hypoxic environment is likely to affect the fate of chondrocytes in the growth plate of A10ΔEC mice, as these cells require proper oxygenation to undergo apoptosis.44 Interestingly, femur length is normal in adolescent 20-day old A10ΔEC mice, which is consistent with this hypothesis. An alternative explanation to the observed shortened femoral length is that hematopoiesis in the femur could lead to more severe hypoxia of its growth plate than in other bones, although this explanation seems unlikely because the bone marrow in A10ΔEC mice contains substantially less hematopoietic cells than the bone marrow in controls. These results support the notion that abnormal vascularization in developing long bones can make a significant contribution to the pathogenesis of phocomelia in humans,45 although additional studies will be required to understand the cause of the shortened femurs in A10ΔEC mice.
The splenic extramedullary hematopoiesis and peripheral reticulocytosis in A10ΔEC mice is presumably secondary to peripheral hemolysis, a notion that is supported by the observation that hematocrit, hemoglobin and red blood cell count are lower than in controls. However, because Notch has been implicated in the regulation of apoptosis in erythroblasts,46 and hematopoietic cells derive from aortic endothelial cells,47 and express Tie2-Cre,48 it is possible that the inactivation of Adam10 by Tie2-Cre also reduces apoptosis in erythroblasts, thereby contributing to the increased number of reticulocytes in A10ΔEC mice. However, there would have to be an additional accelerated hemolysis to explain why other measurements related to red blood cells are decreased. Further studies will be necessary to distinguish between these possible causes.
The viability of A10ΔEC mice provided the unique opportunity to study how the lack of Adam10 in endothelial cells affects pathologic neovascularization in a mouse model for oxygen-induced retinopathy (OIR), which depends on VEGF-A expression triggered through relative hypoxia.33,34 OIR elicited a strong increase in the number of neovascular endothelial cells that crossed the internal limiting membrane in A10ΔEC mice, suggesting that Adam10 normally limits the development of pathologic neovascular tufts. Previous studies of OIR in zebrafish also identified an increase in neovascularization in the presence of γ-secretase inhibitors, which block Notch signaling.49 Interestingly, the endothelial cells that had crossed the internal limiting membrane in retinas of A10ΔEC mice after OIR formed large sheets with lumens containing red blood cells, whereas pathologic neovascularization normally manifests itself in the form of small neovascular tufts that often lack a lumen. These large vein-like structures could be caused by an inability of Notch to limit vein formation6,8 in the absence of Adam10. Finally, the increased revascularization of the central avascular area in A10ΔEC mice suggests that Adam10 also serves to restrict beneficial neovascularization.
In summary, the inactivation of Adam10 in endothelial cells led to a variety of vascular abnormalities, including an increased vascular density at the leading edge of the developing retinal vasculature, enhanced pathologic neovascularization in the OIR model, abnormal vessels in the liver, epicardium and diaphragm, increased endothelial cells in glomeruli and intestinal polyps and an abnormal growth of long bones. Because Adam10 has emerged as a crucial regulator of Notch signaling in a variety of developmental systems, and because Notch is known to regulate cell fate decisions, we hypothesize that the Adam10/Notch signaling axis is crucial for the proper development and/or maintenance of the specialized endothelial cells populating the organ-specific vascular niches that are abnormal in A10ΔEC mice.40 Of the 4 mammalian Notch receptors, Notch1 and Notch4 are highly expressed in endothelial cells.2 It is therefore possible that interruption of signaling through one or both of these receptors is responsible for the multisystemic vascular pathology in A10ΔEC mice, although a role for Adam10 in processing Notch4 remains to be established. This hypothesis would provide a simple and unifying explanation for the various distinct vascular defects in A10ΔEC mice, although we cannot rule out that processing of other substrates by Adam10,50 or other roles of Adam10 that are not related to its catalytic activity, contribute to these vascular phenotypes. Because the Notch1ΔEC mice5 were generated with a Tie2-Cre driver that is known to have germ line expression,36 it will now be interesting to delete Notch1 in endothelial cells with the Tie2-Cre driver used here, and to generate Notch1ΔEC mice that also lack Notch4 to assess vascular development and pathologic neovascularization in these animals. Moreover, it will be interesting to generate mice lacking both Adam10 and Adam17, which has also been implicated in Notch processing,20 in endothelial cells to determine whether Adam17 can perhaps partially compensate for the loss of Adam10 in these cells. Finally, from a clinical perspective, these findings have implications for the use of metalloproteinase inhibitors or Notch inhibitors to treat cancer or proliferative retinopathies, which should be tested for their effect on Adam10 and Notch signaling in endothelial cells to avoid any increase in neovascularization or other unintended consequences on the organ systems affected in A10ΔEC mice.
The online version of TWs article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Mark Rosenblatt for helpful discussions and use of his microscopy facility, Dr Tom Sato for providing Tie2-Cre mice, and Francesca Cardello and Elin Mogollon for their assistance in this project.
This work was supported by EY015719 to C.P.B., and was conducted in a facility constructed with support from Research Facilities Improvement Program Grant C06-RR12538-01 from the National Center for Research Resources, National Institutes of Health. K.G. was supported by a training grant from the National Heart, Lung, and Blood Institute (NHLBI; T32 GM007739-31S1) to the Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD/PhD program. P.S. was supported by the Deutsche Forschungsgemeinschaft SFB877, and IUAP P6/58 of the Belgian Federal Science Policy Office and the Center of Excellence “Inflammation at Interfaces.” B.D.S. is supported by a Methusalem grant (KU Leuven) of the Flemisch Government.
National Institutes of Health
Authorship
Contribution: K.G. performed experiments, analyzed and interpreted the data, and wrote the paper; S.M. performed the histopathologic evaluations and contributed to writing the paper; K.M performed the analysis of embryonic angiogenesis and contributed to writing the paper; B.D-S. and P.S. contributed to the generation of Adam10flox mice and provided intellectual input and corrections on the paper; and C.P.B. supervised the project, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Carl P. Blobel, Arthritis and Tissue Degeneration Program, Caspary Research Bldg, Rm 426, Hospital for Special Surgery, 535 E 70th St, New York, NY 10021; e-mail: blobelc@hss.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal